Review

Open versus laparoscopic colectomy for colorectal cancer: A meta-analysis
1Rajesh Kumar Singh, 2Mridula Shukla, 1Manoj Pandey
- 1Department of Surgical Oncology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, India
- 2Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, India
- Submitted: October 8, 2012
- Accepted: October 19, 2012
- Published: October 24, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Despite a decade experience of surgery by laparoscopy for colorectal cancer the safety and efficacy is still being debatable. We performed a meta-analysis of randomized controlled trials (RCTs), published between 1996 and July 2010 comparing laparoscopic vs open colonic resections for colorectal cancer.
Methods
A thorough search of the Medline, Embase and Cochrane library was made and identified RCTs were obtained and analyzed for short-term and long-term outcome variables. For continuous data mean difference and inverse variance were used for calculating the odds ratio (OR). Z test was used to calculate the overall effect and heterogeneity was tested by Chi-square and I2 values.
Results
A total of 43 studies were identified from 41 RCTs, not all variables were available in all studies, hence only those studies with particular variables were used for individual meta-analysis. A total of 35 studies evaluated 8940 participants with cumulative OR for operating time was 42 (95% CI 38.3 – 45.7); blood loss was evaluated in 19 studies with 5207 participants and an OR of 144.36 (95% CI 149.2-139.5) and 26 studies with 6460 participants reported on hospital stay with OR of 3.26 (95% CI 3.51 – 3.01) while lymph node yield was reported in 16 studies with 5515 participants and OR of 0.24 (0.82 – 0.34) respectively; all favoring laparoscopy. The meta analysis of present data suggest that laparoscopic resection of malignant esophageal or colonic carcinoma may be a better alternative as it has better short term outcome with no difference in survival at 5 years.
Conclusion
The results of present meta-analysis suggests that despite having a higher operating time, the blood loss and the hospital stay is significantly lower in laparoscopic group. The lymph node yield shows a trend favoring laparoscopy, however the effect is not significant. Laparoscopic colectomy appears to be a better method of surgery for colorectal cancer.
Key words
Colon cancer, Colorectal carcinoma, Laparoscopy.
Introduction
Laparoscopic surgery for colorectal cancer has been controversial from the beginning of its first introduction in late 1990s [1]. Since then several of the prospective randomized controlled trials have demonstrated higher efficacy with regards to the short term outcomes like shorter hospital stay, reduced analgesic use, and earlier recovery to bowel movements [2-15]; however, the mean operating time and the average blood loss is higher while the number of lymph nodes retrieved has not been shown to differ significantly [3, 5,6, 9, 11, 13, 16-43]. With regards to the long term survival data, again no difference in survival has been found with 3 years [16, 14, 22] and 5 years follow-up [3, 16-17, 22-24]. Thus, a meta-analysis appears to be the best way to recognize the congruity of these trials and studies.
Material and Methods
Search strategy
Randomized controlled trials (RCTs) of all the published data comparing laparoscopic and open surgery for colorectal cancer were searched in the electronic databases including Cochrane Library, MEDLINE, EMBASE, Pubmed and Google scholar for the years 1996–July 2010. Searches were carried out using medical subject headings (MeSH) and free text words in combination with the search strategy for randomized controlled trials described by Dickersin et al., [26]. The following search was adapted for each database: laparoscopy [MeSH], surgery [MeSH], colon [MeSH], colectomy [MeSH], open [MeSH], colonic neoplasms [MeSH],colon cancer [MeSH], colorectal cancer [MeSH], and rectal neoplasms [MeSH].
All non-randomized studies and unpublished data were excluded. Trials reported in language other than in English were also excluded.
Data extraction
The following data were collected in all randomized controlled trials: neoadjuvant therapy, tumor localization (Right/Transverse/Left colon, rectum), operating time, blood loss, hospital stay, time to pass flatus, time and duration of analgesic use, time to start oral feeds, type of resection (right-sided, left-sided, rectal, abdominoperineal excision, extended colectomy, others), tumor stage (Dukes A,B,C and TNM I–IV), depth of invasion of bowel wall, grade of differentiation, number of lymph nodes harvested, pathological N stage, resection margins including distal and radial margins, complication rates, leakage rates, wound infection, morbidity and mortality rates, recurrence rates, mean follow-up , overall survival, disease free survival, adjuvant therapy.
Assessment of methodological quality
- Review by MP, MS.
- Data analysis based on the ‘intention-to-treat’-principle.
Statistical analysis
The data regarding the short term and long term variables were extracted and entered in the Revman 5 software. For continuous data inverse variance method using mean difference was used to calculate the odds ratio and 95% CI using either random or fixed effect. For dichotomous variables odds ratios with their 95% confidence intervals were calculated. Analyses were performed using fixed effects models. Due to the large number of small studies that were included, these analyses were compared with analyses using random effects models. Heterogeneity was tested using Chi square and I2 test. Significance was tested with Z test.
Result
A total of 61 published papers were identified. Of these 20 were excluded because 1 included benign diseases (Inflammatory bowel disease, polyps); one included advanced colorectal cancer; two were repetitions; and in 16 articles relevant data could not be extracted. Thus a total of 41 articles of RCTs were identified containing 43 studies. All of the included RCTs were published as full articles.
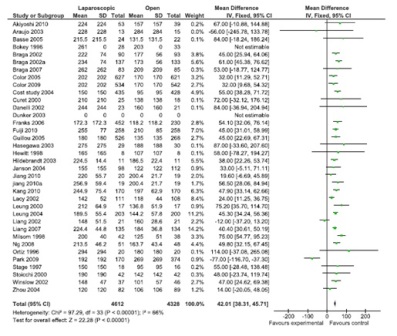
Figure 1: Operating time (36 studies, 8940 participants, favor open surgery).
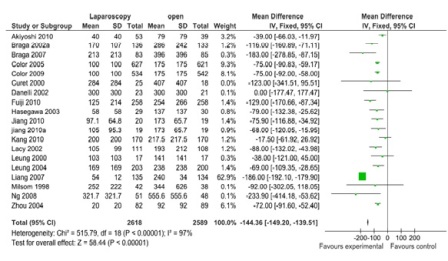
Figure 2: Blood loss (19 studies, 5207 participants; favors laparoscopy).
A total of 43 comparisons were identified from 41 RCTs, not all variables were available in all studies, and hence only those studies with particular variables were used for individual meta-analysis. Data on operating time was extracted from a total of 35 studies comprising 8940 participants. The mean/median operating time ranged from 120-256 minutes in; laparoscopy arm compared to 95-269 minutes in open surgery (Figure 1). The cumulative odds ratio was 42 with 95% CI of 38.3 – 45.7 favoring open surgery. Blood loss was evaluated in 19 studies with 5207 participants and cumulative OR of 144.36 favoring laparoscopy (95% CI 149.2-139.5). The average blood loss during Laparoscopic surgery was 20-320 ml versus 79-555 ml in open arm respectively (Figure 2). Hospital stay was studied in 26 studies with 6460 participants reported an OR of 3.26 (3.51 – 3.01) again favoring laparoscopy (Figure 3). Lymph node yield was reported in 16 studies with 5515 participants, an odds ratio -0.24 (0.82 – 0.34) was observed favoring laparoscopic procedures, although not clinically significant (Figure 4). Data from three studies including 2733 participants revealed an odds ratio of 0.97(0.8-1.17) showing no significant difference in survival at three years of follow up (Figure 5). A total of 2966 participants from 5 studies showed an equivocal 5 years of survival between the two arms.(Odds ratio of 1.08 and 95% CI 0.9-1.28) (Figure 6).
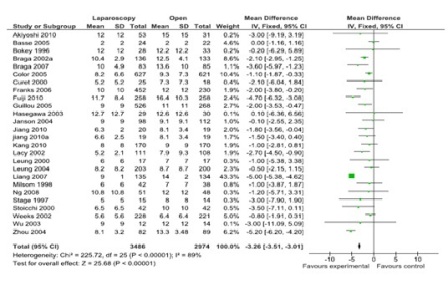
Figure 3: Hospital stay (26 studies, 6460 participants; favor laparoscopy).
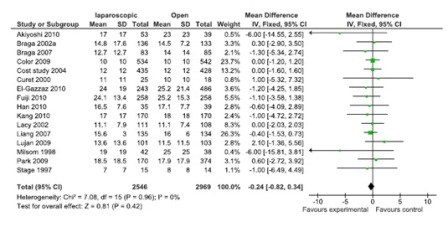
Figure 4: Lymph node yield (16 studies, 155 participants; No significant difference).
Discussion
The introduction of Laparoscopic technique has been enthusiastically followed to the resection of colorectal cancer for more than 10 years. As of today, most of the colorectal surgeons routinely use the laparoscopic approach and attribute the advantages to the quicker functional recovery and comparable oncologic results. Till of date many trials have reported many benefits of the laparoscopic method, including the short term and the long term aspects. Short term benefits include reduction in surgical stress and immunosuppression, less intraoperative blood loss, less postoperative pain and the requirement of analgesia, early recovery of bowel function in terms of flatus and feces, reduced hospital stay, and faster recovery to perform normal activities [15, 44-47]. This is also an important confirmatory finding that provides support for the laparoscopic approach in terms of long-term survival by confirming from a surgical standpoint that the laparoscopic approach provides as good a resection as the open approach for treating CRC. But the feasibility of the technique will only be established if the long term outcomes are equally comparable and acceptable. Some of the studies have also concluded that laparoscopy may even be detrimental to the patient with a higher recurrence and mortality rates [21, 48, 49]. The operative time was significantly longer with laparoscopic cancer surgery as compared with open surgery; a finding that has been consistent in laparoscopic colorectal surgery. Postoperative recovery was significantly better for patients who underwent laparoscopic resection in terms of bowel function and shortened hospital stay. This was also true for abdominoperineal resections, for which the requirement for parenteral analgesia was also significantly reduced in the laparoscopic group. It is important to note that time to feeding solids is a measure of recovery that is prone to bias, because surgeons with an interest in laparoscopic cancer surgery are likely to feed their patients earlier than for open rectal cancer surgery. Time to first bowel movement is a better measure, but it may also be affected by the decision to commence oral intake. Improvement in the return of bowel function and reduced hospital stay has been shown by both the COST and Conventional versus Laparoscopic-Assisted Surgery in patients with Colorectal Cancer (CLASICC) trials for laparoscopic colorectal cancer surgery, although neither has demonstrated an improvement in quality of life. The results of this meta-analysis of patients have shown that there was no significant difference in the proportion of patients with the ‘‘number of lymph nodes harvested’’ when considering the laparoscopic groups. This finding suggests that specimens from laparoscopic and open surgery were comparable in terms of their adequacy of resection and oncological clearance. Because these operative factors strongly influence the likelihood of curative resection and ultimately, survival after laparoscopic surgery for colorectal cancer, this is an important confirmatory finding. The presented meta-analysis shows that long term results in terms of 3 or 5 year survival are not different by two approaches. It should be noted that these studies has some limitations. Since patients who were lost to follow-up are excluded, thereby lowering both the power and the validity of the studies. This could cause a bias. Secondly, we did not perform a separate analysis comparing converted procedures, completed laparoscopic procedures, and open surgery procedures for CRC, since this analysis would be biased. Thirdly, it was not possible to match all patient groups for tumor grade, stage, and adjuvant treatment, all of which are factors known to affect outcome for patients with rectal cancer. This study could not account for the learning curve associated with the technique and its effect on postoperative outcome, and this is an important consideration because it has been suggested that laparoscopic resection of the rectum is more technically demanding and is associated with a longer learning curve than other laparoscopic colonic resections.
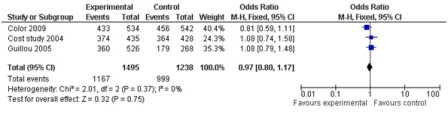
Figure 5: 3 year overall survival (3 studies; 2733 participants; no significant difference).
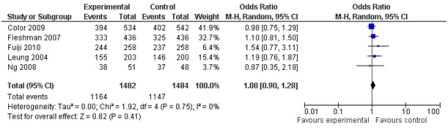
Figure 6: 5 year overall survival (5 studies, 2966 participants; no statistically significant difference).
Conclusion
The meta analysis of present data suggest that laparoscopic resection of malignant colonic carcinoma may be a better alternative as it has better short term outcome with no difference in survival at 5 years. Therefore, successful laparoscopic colorectal resection for CRC is as effective as open surgery in terms of oncological outcomes, and these results support the continued use of laparoscopic surgery in patients with CRC.
Authors' Contribution
RKS: Did the literature search and prepared the manuscript.
MS: Literature search, analysis and manuscript preparation.
MP: Concept, Design, Analysis and interpretation of data.
Conflict of Interests
The authors declare that there are no conflicts of interests.
Funding
None
Acknowledgement
This article was presented in the Annual conference of Indian Association of Surgical Oncology 2011.
References
[1]. Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991; 1:144–150 [Pubmed]
[2]. Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010; 11: 637–45.[Pubmed]
[3]. Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer; a randomized trial. Lancet 2002; 359: 2224–9.[Pubmed]
[4]. Leung KL, Lai PB, Ho RL, Meng WC, Yiu RY, Lee JF, Lau WY. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma. Ann Surg 2000;231: 506–511.[Pubmed]
[5]. Milsom JW, Böhm B, Hammerhofer KA, Fazio V, Steiger E, Elson P. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg. 1998; 187(1):46-54; discussion 54-5.[Pubmed]
[6]. Akiyoshi T, Kuroyanagi H, Fujimoto Y, Konishi T, Ueno M, Oya M, Yamaguchi T. Short-term outcomes of laparoscopic colectomy for transverse colon cancer. J Gastrointest Surg. 2010; 14(5):818-23.[Pubmed]
[7]. Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Di Carlo V. Laparoscopic versus open colorectal surgery. A randomized trial on short-term outcome. Ann Surg 2002; 236:759–767.[Pubmed]
[8]. Danelli G, Berti M, Perotti V, Albertin A, Baccari P, Deni F, Fanelli G, Casati A.; Temperature control and recovery of bowel function after laparoscopic or laparotomic colorectal surgery in patients receiving combined epidural/general anesthesia and postoperative epidural analgesia. Anesth Analg 2002; 95: 467–471.[Pubmed]
[9]. Jiang JK, Chen WS, Wang SJ, Lin JK. A novel lifting system for minimally accessed surgery: a prospective comparison between "Laparo-V" gasless and CO2 pneumoperitoneum laparoscopic colorectal surgery. Int J Colorectal Dis. 2010; 25(8):997-1004.[Pubmed]
[10]. Schwenk W, Böhm B, Haase O, Junghans T, Müller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Arch Surg. 1998; 383(1): 49-55.[Pubmed]
[11]. Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng Z, Li L, Shu Y, Wang TC. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc 2004; 18: 1211–5.[Pubmed]
[12]. Stage JG, Schulze S, Moller P, Overgaard H, Andersen M,Rebsdorf-Pedersen VB, Nielsen HJ. Prospective randomized study of laparoscopic versus open colonic resection for adenocarcinoma.Br J Surg 1997; 84:391–396.[Pubmed]
[13]. Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomized trial. Lancet 2004; 363:1187–1192.[Pubmed]
[14]. Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM, MRC CLASICC Trial Group. Short-term endpoints of conventional versus laparoscopic assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomized controlled study. Lancet 2005; 365: 1718–1726.[Pubmed]
[15]. The Colon Cancer, Laparoscopic or Open Resection Study Group. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomized trial. Lancet Oncol 2005; 6:477–484.[Pubmed]
[16]. COLOR Study Group; Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomized clinical trial. Lancet Oncol 2009; 10: 44–52.[Pubmed]
[17]. Fujii S, Ota M, I chikawa Y, Yamagishi S, Watanabe K, Tatsumi K, Watanabe J, Suwa H, Oshima T, Kunisaki C, Ohki S, Endo I, Shimada H. Comparison of short, long-term surgical outcomes and mid-term health-related quality of life after laparoscopic and open resection for colorectal cancer: a case-matched control study. Int J Colorectal Dis. 2010; 25(11): 1311-23.[Pubmed]
[18]. Hasegawa H, Kabeshima Y, Watanabe M, Yamamoto S, Kitajima M. Randomized controlled trial of laparoscopic versus open colectomy for advanced colorectal cancer. Surg Endosc. 2003;17(4):636-40.[Pubmed]
[19]. Liang JT, Huang KC, Lai HS, Lee PH, Jeng YM. Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann Surg Oncol 2007;14:109–17.[Pubmed]
[20]. Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Teoh AY, Leung WW. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008; 15(9): 2418–25.[Pubmed]
[21]. Curet MJ, Putrakul K, Pitcher DE, Josloff RK, Zucker KA. Laparoscopically assisted colon resection for colon carcinoma: perioperative results and long-term outcome. Surg Endosc. 2000; 14(11):1062-6.[Pubmed]
[22]. The COST Group. A comparison of laparoscopically-assisted and open colectomy for colon cancer. N Engl J Med 2004; 350: 2050–59.[Pubmed]
[23]. Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Di Carlo V. Laparoscopic versus open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum 2005; 48: 2217–2223.[Pubmed]
[24]. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST study group trial. Ann Surg 2007; 246:655– 664.
[25]. Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994; 309: 1286–91.[Pubmed]
[26]. Araujo SE, da Silva eSousa AH Jr, de Campos FG, Habr-Gama A, Dumarco RB, Caravatto PP, Nahas SC, da Silva J, Kiss DR, Gama-Rodrigues JJ. Conventional approach x laparoscopic abdominoperineal resection for rectal cancer treatment after neoadjuvant chemoradiation: results of a prospective randomized trial. Rev Hosp Clin Fac Med Sao Paulo. 2003; 58(3):133-40.[Pubmed]
[27]. Basse L, Jakobsen DH, Bardram L, Billesbølle P, Lund C, Mogensen T, Rosenberg J, Kehlet H. Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg. 2005;241(3):416-23.[Pubmed].
[28]. Bokey EL, Moore JW, Chapuis PH, Newland RC. Morbidity and mortality following laparoscopic-assisted right hemicolectomy for cancer. Dis Colon Rectum. 1996; 39(10 Suppl):S24-8.[Pubmed]
[29]. Dunker MS, Ten Hove T, Bemelmann WA, Slors JF, Gouma DJ, van Deventer SJ. Interleukin-6, C-reactive protein, and expression of human leukocyte antigen-DR on peripheral mononuclear cells in patients after laparoscopic vs. conventional bowel resection. Dis Colon Rectum 2003;46:1238–1244.[Pubmed]
[30]. El-Gazzaz G, Hull T, Hammel J, Geisler D. Does a laparoscopic approach affect the number of lymph nodes harvested during curative surgery for colorectal cancer? Surg Endosc. 2010;24(1):113-8.[Pubmed]
[31]. Franks PJ, Bosanquet N, Thorpe H, Brown JM, Copeland J, Smith AM, Quirke P, Guillou PJ; CLASICC trial participants. Short-term costs of conventional vs laparoscopic assisted surgery in patients with colorectal cancer (MRC CLASICC trial). Br J Cancer. 2006;95(1):6-12.[Pubmed]
[32]. Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H; for The Clinical Outcomes of Surgical Therapy Study Group. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007; 246(4):655-62; discussion 662-4.[Pubmed]
[33]. Han SA, Lee WY, Park CM, Yun SH, Chun HK. Comparison of immunologic outcomes of laparoscopic vs open approaches in clinical stage III colorectal cancer. Int J Colorectal Dis. 2010; 25(5):631-8.[Pubmed]
[34]. Hewitt PM, Ip SM, Kwok SP, Somers SS, Li K, Leung KL, Lau WY, Li AK. Laparoscopic-assisted vs. open surgery for colorectal cancer :comparative study of immune effects. Dis Colon Rectum 1998;41:901–9.[Pubmed]
[35]. Hildebrandt U, Kessler K, Pistorius G, Menger, MD, et al.,. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc 2003;17:242–246.[Pubmed]
[36]. Janson M, Bjorholt I, Carlsson P, Haglind E, Henriksson M, Lindholm E, Anderberg B. Randomized clinical trial of the costs of open and laparoscopic surgery for colonic cancer. Br J Surg 2004; 91: 409–417.[Pubmed]
[37]. Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007; 25(21):3061-8.[Pubmed]
[38]. Liang JT, Shieh MJ, Chen CN, Cheng YM, Chang KJ, Wang SM. Prospective evaluation of laparoscopy-assisted colectomy versus laparotomy with resection for management of complex polyps of the sigmoid colon. World J Surg 2002; 26: 377–383.[Pubmed]
[39]. Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg 2009; 96: 982–89.[Pubmed]
[40]. Ortiz H, Armendariz P, Yarnoz C. Early postoperative feeding after elective colorectal surgery is not a benefit unique to laparoscopy assisted procedures. Int J Colorect Dis 1996;11:246–249.[Pubmed]
[41]. Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Laparoscopic resection of extraperitoneal rectal cancer: a comparative analysis with open resection. Surg Endosc. 2009; 23:1818–24.[Pubmed]
[42]. Stocchi L, Nelson H, Young-Fadok TM, Larson DR, Ilstrup DM. Safety and advantages of laparoscopic vs. open colectomy in the elderly: matched-control study. Dis Colon Rectum 2000; 43:326–332.[Pubmed]
[43]. Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparocopic vs open colectomy. Surg Endosc 2002; 16(10):1420–5.[Pubmed]
[44]. Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G, et al.,. Short-term quality-of-life outcomes following laparoscopic assisted colectomy vs.open colectomy for colon cancer: a randomized trial. JAMA 2002; 287:321–328.[Pubmed]
[45]. Wu FP, Sietses C, von Blomberg BM, van Leeuwen PA, Meijer S, Cuesta MA. Systemic and peritoneal inflammatory response after laparoscopic or conventional colon resection in cancer patients. Dis Colon Rectum 2003;46:147–155.[Pubmed]
[46]. Abraham NS, Young JM, Solomon MJ. Meta-analysis of shortterm outcomes after laparoscopic resection for colorectal cancer.Br J Surg. 2004;91:1111–24.[Pubmed]
[47]. Franklin ME Jr, Rosenthal D, Abrego-Medina D, Dorman JP,Glass JL, Norem R, Diaz A. Prospective comparison of open vs.laparoscopic colon surgery for carcinoma. Five-year results. DisColon Rectum. 1996;39:S35-46 [Pubmed]
[48]. Phillips EH, Franklin M, Carroll BJ, Fallas MJ, Ramos R, Rosenthal D. Laparoscopic colectomy. Ann Surg. 1992;216:703–7.[Pubmed]
[49]. Kehlet H. Surgical stress response: does endoscopic surgery confirm an advantage? World J Surg. 1999;23:801–7.[Pubmed]

