Case Report

Collision tumor of the thyroid: follicular carcinoma plus papillary carcinoma plus adenomatous goiter
1Kenichi Takano, 2Keisuke Kikuchi, 3Hiroshi Matsumiya, 1Tetsuo Himi
- 1Department of Otolaryngology, Sapporo Medical University School of Medicine, Sapporo, Japan
- 2Department of Pathology, Obihiro Kosei General Hospital, Obihiro, Japan
- 3Department of Otolaryngology, Obihiro Kosei General Hospital, Obihiro, Japan
- Submitted: March 01, 2013;
- Accepted: March 19, 2013;
- Published: April 02, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The combination of three different types of collision tumors in a single thyroid gland is an unusual event. The term “collision tumor” represents the coexistence of two or more histologically distinct tumors in close proximity to each other.
Case report
We report the case of a 78-year-old female who presented with a cervical swelling. Histopathological findings revealed a true “collision tumor” with components of both follicular and papillary carcinomas.
Discussion
Whether the etiology of neoplastic transformation istumorgenic stimuli or the collision phenomenon remains controversial.
Key words
Collision tumor, Thyroid carcinoma, Histopathology.
Introduction
The combination of three different types of collision tumors in a single thyroid gland is an unusual event. The term “collision tumors” refers to two or more coexistent but independent tumors and these tumors are distinct from composite or mixed tumors [1]. Although collision tumors of the thyroid have been described [2-5], only two reports have addressed combinations of three different tumors [6, 7]. We present the case of a patient with thyroid collision tumors that were a combination of follicular and papillary carcinomas and adenomatous goiter.
Case Report
A 78-year-old Japanese female presented with an anterior cervical swelling. There was no family history of any endocrine or non-endocrine malignant tumors. Her personal history included hypertension and hyperlipidemia. Physical examination revealed a single nodule, approximately 1 cm in diameter, over the right lobe and the left lobe of the thyroid, respectively. Her vocal cords were mobile. A routine ultrasound examination and computed tomogram of the neck revealed two calcified nodules in the right lobe of the thyroid, and heterogeneous tumor of the left lobe. Fine needle aspiration (FNA) biopsy findings were consistent with papillary thyroid carcinoma. The findings of thyroid function tests (T3, T4, and TSH) were within the normal limits, and the serum thyroglobulin level was elevated. The patient underwent a total thyroidectomy. At surgery, the tumor was confirmed to originate from the thyroid gland. She has been continually examined every 3 months and has been tumor free for 2 year after the thyroidectomy.
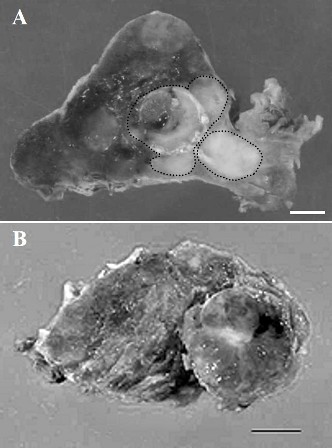
Figure 1: (A) Cut surface of specimen showing a solid follicular carcinoma (left, surrounded by dotted line) and an adjoining homogenous white papillary carcinoma (right, surrounded by dotted line). Bar = 0.5 cm. (B) Cut surface of specimen showing adenomatous goiter (surrounded by dotted line). Bar = 0.5 cm.
Macroscopically, the right hemithyroidectomy specimen measuring 5.0 × 4.0 × 2.5 cm was a solid encapsulated tumor involving the right lobe and the isthmus. On the cut surface, a gray-white fleshy partially calcified tumor measuring 1.5 cm at the greatest dimension was observed within the thyroid parenchyma (Figure 1A). Another distinct gray-white tumor measuring 1.0 cm at the greatest dimension was present juxtaposed to this tumor (Figure 1A). On the other hand, the left hemithyroidectomy specimen measuring 5.0 ×3.0 ×2.0 cm revealed a multinodular pattern with cystic changes and small calcifications (Figure 1B).
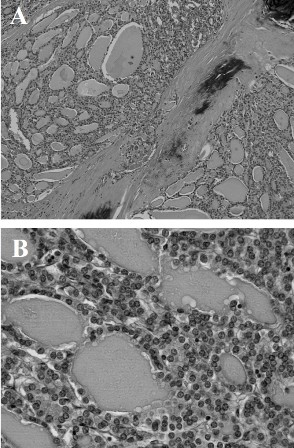
Figure 2: (A) Low-power view of follicular carcinoma showing the follicular structure (hematoxylin-eosin; original magnification, ×100). (B) High-power view of follicular carcinoma (hematoxylin-eosin; original magnification, ×400).
Microscopically, multiple sections from the right lobe of the thyroid displayed an invasive follicular carcinoma with foci of transcapsular invasion. No obvious extra thyroidal extension of the tumor was noted. In opposition to the follicular carcinoma, there was an independent primary tumor that exhibited the histological findings of a papillary carcinoma. The two tumors were separated by fibrous septa. The follicular carcinoma showed uniform follicular differentiation with a broad fibrous capsule and infiltrative growth. The follicular carcinoma cells had round to oval nuclei with fine chromatin, and they were arranged in a solid and micro follicular pattern (Figure 2). The papillary carcinoma demonstrated invasive growth with fibrosis, and the nuclei of the tumor cells were larger and oval and had typical open clear chromatin and nuclear holes and grooves (Figure 3). On the other hand, the adenomaotus goiter of the right lobe showed the presence of many follicles of different sizes (Figure 4). These three tumors were morphologically and histologically distinct and were also independent, i.e., a zone of transition from follicular to papillary carcinoma was absent, representing a true “collision tumor.”
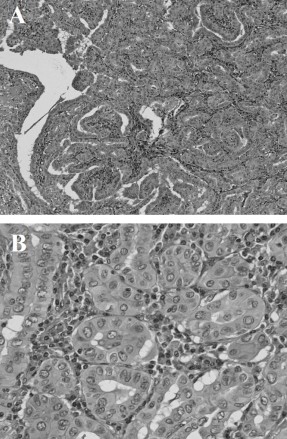
Figure 3: (A) Low-power view of papillary carcinoma (hematoxylin-eosin; original magnification, ×100). (B) High-power view of papillary carcinoma showing ground-glass nucleus, nuclear groove, and nuclear pseudo inclusion (hematoxylin-eosin; original magnification, ×400).
Discussion
The term “collision tumor” refers to multiple coexistent but independent tumors that are histologically distinct [1]. Collision tumors of the thyroid are distinct from composite and mixed tumors. Composite tumors contain two discrete cell populations consisting of thyroglobulin-positive papillary carcinoma cells and calcitonin-positive medullary carcinoma cells [2]. On the other hand, mixed tumors have a common cellular origin, and their cells co express both thyroglobulin and calcitonin [2]. Several hypotheses have been described as the mechanisms underlying collision tumors [2, 4].The first hypothesis is a “coincidental colocalization” of the multiple primary tumors. Multiple different tumors may contiguously develop in a region altered by the same carcinogenic stimuli. Another hypothesis suggests that the presence of the first tumor alters the microenvironment, thereby increasing the probability of a second adjacent tumor. In fact, it has been reported that certain pairs of collision tumors occur more frequently, e.g., hepatocellular carcinoma with cholangio carcinoma [8, 9] and gastric adenocarcinoma intermixed with gastric lymphoma [10, 11]. The third hypothesis suggests that the multiple tumors originate from a common stem cell [2, 4]. In the thyroid, combinations of papillary and medullary thyroid carcinoma or other neoplasms have been reported [1-5, 12], however the combination of three different types of thyroid tumor were rare [7]. These tumors have originated from either a coincidental meeting or a common stem cell. Although papillary thyroid carcinoma is not rare, occurring in 18%–22% of thyroid neoplasms, the presence of a morphologically and histogenetically different primary neoplasia in the thyroid gland is very rare.
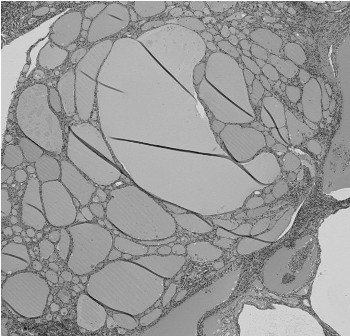
Figure 4: Histological findings of adenomatous goiter, showing the presence of many follicles of different sizes (hematoxylin-eosin; original magnification, ×40).
Several genes and genetic alterations that are involved in the tumor genesis of each type of thyroid carcinoma have been identified. Fukushima et al. found that BRAF mutations were exclusively detected in papillary thyroid carcinomas at a frequency of 30%–70% [13]. It has also been reported that somatic rearrangements of RET/PTC in papillary thyroid carcinoma are highly prevalent [14]. In thyroid follicular carcinomas, 2 known initiating events are RAS mutations and PAX8-PPARγ rearrangements; RAS predisposes to the dedifferentiation of follicular carcinomas [15, 16].Today, no common mutation is known to be involved in the pathogenesis of both papillary and follicular carcinomas.
Recently, Takano proposed a new model of thyroid carcinogenesis that was termed the “fetal cell carcinogenesis” model [17]. According to the multi-step carcinogenesis model, which is generally accepted, thyroid carcinomas are generated from thyrocytes via multiple genomic changes that foster the development of cancerous characteristics. On the other hand, based on the fetal cell carcinogenesis model, thyroid carcinomas are derived from the remnants of fetal thyroid cells that have the ability to migrate to surrounding tissues [17]. In this hypothesis, papillary carcinomas, follicular tumors, and anaplastic carcinomas are thought to be derived from the remnants of thyroblasts, prothyrocytes, and thyroid stem cells, respectively [17]. Because follicular cells are formed at the very end of differentiation, follicular tumors can originate in various ways from several different cells, including thyroid stem cells, thyroblasts, and prethyrocytes; on the other hand, papillary carcinomas are almost always directly derived from thypoblasts. Accordingly, the follicular carcinoma in this case might have originated from the papillary carcinoma, which developed first, or both tumors may have originated from a common stem cell.
In conclusion, the combination of three different types of collision tumors in the thyroid is an unusual event and presents a diagnostic and therapeutic challenge. The treatment of collision tumors should depend on the combination of primary tumors involved, and each component of the combination should be treated as an independent primary tumor. The reporting of similar cases with longer follow-up periods will help define the epidemiology and biology of these extremely rare tumors and establish standardized protocols for their treatment.
Author’s Contribution
KT: Did the literature search and prepared the draft manuscript.
HM: Did the literature search and helped in preparation of manuscript.
KK: Helped with the pathological part of manuscript.
TH: concept and design, review and revision of manuscript.
Conflict of Interests
The authors declare that there are no conflicts of interest.
Ethical Considerations
Written consent was obtained from the patient for publication of this case report.
Funding
None
Reference
[1]. Lee AH, Soomro IN. Collision tumour of the pleura composed of small cell carcinoma and malignant mesothelioma. Histopathology 2004; 45: 305-306. [Pubmed].
[2]. Baloch ZW, Mandel S, LiVolsi VA. Combined tall cell carcinoma and Hürthle cell carcinoma (collision tumor) of the thyroid. Arch Pathol Lab Med 2001; 125: 541-543. [Pubmed].
[3]. Wu CJ, Chen HL, Song YM, et al. Mixed medullary-follicular carcinoma and papillary carcinoma of the same thyroid. Intern Med 1998; 37: 955-957. [Pubmed].
[4]. Walvekar RR, Kane SV, D'Cruz AK. Collision tumor of the thyroid: follicular variant of papillary carcinoma and squamous carcinoma. World J Surg Oncol 2006; 19: 65. [Pubmed].
[5]. Gero MJ, Lipper S, Chernys AE, et al. Medullary and papillary carcinomas occurring as a collision tumor: report of a case. ClinNucl Med 1989; 14: 171-174. [Pubmed].
[6]. Gonzalez-Campora R, Lopez-Garrido J, Martin-Lacave I, et al. Concurrence of a symptomatic encapsulated follicular carcinoma, an occult papillary carcinoma and a medullary carcinoma in the same patient. Histopathology 1992;21:380-382. [Pubmed].
[7]. Cupisti K, Raffel A, Ramp U, et al. Synchronous occurrence of a follicular, papillary and medullary thyroid carcinoma in a recurrent goiter.Endocr J 2005;52:281-285. [Pubmed].
[8]. Goodman ZD, Ishak KG, Langloss JM, et al. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 1985; 55: 124-135. [Pubmed].
[9]. Haratake J, Hashimoto H. An immunohistochemical analysis of 13 cases with combined hepatocellular and cholangiocellular carcinoma. Liver 1995; 15: 9-15. [Pubmed].
[10]. Goteri G, Ranaldi R, Rezai B, et al. Synchronous mucosa-associated lymphoid tissue lymphoma and adenocarcinoma of the stomach. Am J Surg Pathol 1997; 21: 505-509. [Pubmed].
[11]. Nakamura S, Aoyagi K, Iwanaga S, et al. Synchronous and metachronous primary gastric lymphoma and adenocarcinoma: a clinicopathological study of 12 patients. Cancer 1997; 79: 1077-1085. [Pubmed].
[12]. Pastolero GC, Coire CI, Asa SL. Concurrent medullary and papillary carcinomas of thyroid with lymph node metastases. A collision phenomenon. Am J Surg Pathol 1996; 20: 245-250. [Pubmed].
[13]. Mashiko M, Ohtake T, Endo Y, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene 2003; 25: 6455-6457. [Pubmed].
[14]. Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 2003; 17: 4578-4580. [Pubmed].
[15]. Kroll TG, Sarraf P, Pecciarini L, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma. Science 2003; 25: 1357-1360. [Pubmed].
[16]. Vasko V, Ferrand M, Di Cristofaro J, et al. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab 2003; 88: 2745-2752. [Pubmed].
[17]. Takano T. Fetal cell carcinogenesis of the thyroid: a hypothesis for better understanding of gene expression profile and genomic alternation in thyroid carcinoma. Endocr J 2004; 51: 509-515. [Pubmed].

