Case Report

A Small- and Medium-Vessel Vasculitis-Causing Colo-Colonic Intussusception: A Case Report and Review of the Literature
1Thomas F. Olson, 2Timothy Sorrells, 3Matthew Wilson
- 1Medical Department – US Marine Corps Security Force Regiment, Norfolk, VA, USA;
- 2Department of Pathology, Naval Medical Center Portsmouth, USA;
- 3Department of Surgery, Naval Medical Center Portsmouth, USA
- Submitted: Thursday, April 23, 2015
- Accepted: Thursday, June 25, 2015
- Published: Monday, June 29, 2015
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ((http://creativecommons.org/licenses/by/3.0)which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Intussusception is the invagination of one segment of the gastrointestinal tract into an adjacent segment. In the adult population, intussusceptions confined to the colon which present as a single, initial manifestation of vasculitis are less common.
Case Presentation
We report a rare case of vasculitis of uncertain etiology serving as the lead point for a colo-colonic intussusception in an adult male patient.
Conclusions
Whereas in some cases management of adult intussusception is straightforward, the management of other cases can be controversial. Awareness of the advantages and disadvantages of different management recommendations is important for surgeons. Surgical resection was considered mandatory in our case because of the possibility of occult malignancy serving as the lead point of the intussusception.
Keywords
Intussusception; Colo-colonic; Vasculitis; Florid Vascular Proliferation
Introduction
Intussusception is the invagination of one segment of the gastrointestinal tract into an adjacent segment [1]. In adults, 70–90% of intussusceptions have a defined pathologic lead point [2-5]. Of these cases, neoplastic lead points account for approximately 63% [6], with malignant lesions associated in approximately 50% [7-10]. Of those pathologic lead points confined to the small bowel, malignant neoplasms represent approximately 30% [3, 11], whereas they are more likely to represent up to 66% of malignant neoplasms confined to the large bowel[3, 10, 11, 12].
Less commonly, non-neoplastic vascular abnormalities can form within the bowel wall in association with intussusception. Forms of systemic vasculitis that have been reported in association with intussusception include Henoch-Schonlein purpura (HSP), systemic lupus erythematosus (SLE), polyarteritis nodosa (PAN), enterocolic lymphocytic phlebitis, and Kawasaki’s disease. Additionally, it has been suggested that mechanical and ischemic stress placed on the bowel wall from an intussusception can trigger angiogenesis, resulting in florid vascular proliferations of the colonic wall [13-16].
The management of intussusception in adults can be controversial, and awareness of the advantages and disadvantages of different management recommendations is important for surgeons. We report the first case in the English literature of an adult colo-colonic intussusception as the index manifestation of non-specific vasculitis, followed by a review of the different management recommendations.
Case Presentation
A 32-year-old Caucasian male with a past medical history significant for eczema presented to the hospital with a 1-day history of lower abdominal pain. He described the pain as sharp, intermittent and progressively getting worse since the symptom onset. He denied changes in his bowel and bladder habits. After the development of nausea and vomiting shortly after symptom onset, the patient presented to the emergency department.
Upon initial presentation, the patient was afebrile but mildly tachycardic. Physical examination was notable for tenderness with rebound in the right lower quadrant. Lab results revealed mild leukocytosis (13.6 x 109 cells/L) but were otherwise normal. A plain abdominal computed tomography (CT) scan was performed, which revealed a presumed ileocecal intussusception with evidence of obstruction as well as focal areas of intramural air (Figure 1) The preoperative diagnosis of ileocecal intussusception was made, and the patient was admitted for surgery.
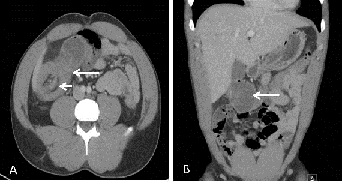
Figure 1: Contrast-enhanced computed tomography scan of the abdomen. (A) Axial image showing the classic target sign (short arrow) as well as invagination of mesenteric fat (long arrow); both signs are consistent with a large bowel intussusception. (B) Coronal image showing multiple concentric signs of the colon (arrow), which is consistent with typical signs of intussusception.
The laparotomy revealed several enlarged and palpable nodes at the base of the mesentery. However, necrotic bowel, purulent fluid, and tissue masses were not found. An intussusception to the hepatic flexure was identified, and a right hemicolectomy was performed. Macroscopic examination of the excised specimen revealed an intramural lesion with mucosal prominence within the cecum. The mucosal lesion appeared to serve as the lead point for the intussusception. Microscopic examination of the mucosal lesion revealed inflammation of numerous small- and medium-sized vessels and fibrinoid changes within the vessel walls (Figure. 2); no neoplastic changes were observed. After additional testing and review of symptoms, the etiology of the vascular lesion remained unclear. Subsequent immunohistochemical staining of the tissue sample was negative for IgA. With the exception of C-reactive protein (mg/L) and erythrocyte sedimentation rate (mm/hr), both of which were elevated with scores over 50 at the time of the intussusception, further laboratory evidence for inflammatory markers, which included a lupus anticoagulant panel, a beta-2 glycoprotein 1 antibody panel, ANA analysis, a complement panel, a cardiolipin panel, a cryoglobulin screen, CCA antibody IgA+IgG analysis, an ANCA panel, rheumatoid factor analysis, and a hepatitis A/B/C panel, was negative or unremarkable. The follow-up interval was 8 months, and no recurrence of symptoms was noted.
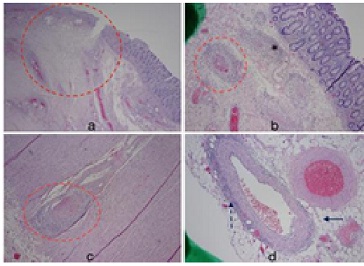
Figure 2: (a) Histopathologic examination of the resected cecum showing polypoid ulcerated bowel mucosa adjacent to normal mucosa. Ischemic changes are noted within hashed circle. (b) Colonic wall showing normal bowel mucosa and submucosal fibrinoid changes with evidence of inflammatory cells surrounding the vessels (hashed circle). Fibrin thrombi can be observed within the vessel (hematoxylin and eosin, ×40). (c) Histopathological examination of the muscularis propria within the colonic wall. The hashed circle shows a vessel with signs of adjacent chronic inflammation and fibrin thrombi within the vessel (hematoxylin and eosin, ×40). (d) Microscopic examination showing the subserosa of the colonic wall. Note the normal appearing adipose tissue (arrow). Additionally, note similar chronic inflammatory and fibrinoid changes of the vessel wall within the subserosa (hashed arrow; hematoxylin and eosin, ×40).
Discussion
Intussusception Associated With Vasculitis
Intussusception Associated With Vasculitis Cases of vasculitis serving as the lead point for intussusception are rare. The mechanism associated with vasculitis-mediated intussusception is not well understood. However, it has been suggested that the affected bowel can undergo altered peristalsis secondary to neuromuscular dysfunction as well as serosal thickening resulting in intussusception. A pertinent literature review revealed multiple adult cases of a systemic vasculitis in association with intussusception, most commonly ileo-colic. However, a vasculitis serving as the lead point in colo-colonic intussusception is even rarer in the adult population. To the best of our knowledge, only three of these cases [17, 18] have been reported in the English literature. Goda
et al., [17] described an adult malewith a purpuric rash on the lower legs, ankles, feet, and peri-anal region prior to presentation with colo-colonic intussusception. The cecal mass which served as the lead point for the intussusception was resected and revealed histologic evidence of diffuse and multiple ulcerations and erosions with lymphocytic and necrotizing vasculitis. Renal biopsy was performed one month later, and immunohistochemical staining of the kidney demonstrated IgA deposition in the mesangium and paramesangium, consistent with HSP. Similarly, Zimmerman
et al., [18] reported two cases in which the cecal lesion serving as the lead point revealed a marked inflammatory reaction of the blood vessels, as well as fibrinoid degeneration of the vessel walls and segmental necrosis. Both patients were diagnosed with PAN, and this correlated with their symptoms prior to presentation which included arthralgias, numbness and cold intolerance to the hands, discrete papules, bullae and ulcers on the extremities, as well as insidious onset of fatigue and weakness. In contrast to these reports, our case did not exhibit definitive pathologic evidence of a specific vasculitis, nor did our patient describe symptoms other than eczema prior to initial presentation. Given the continued lack of systemic features in our patient, the definitive etiology of the small and medium vessel vasculitis remained unclear.
There have also been reports of intussusception associated with other non-neoplastic vascular lesions, to include florid vascular proliferations of the bowel wall. In a report by Bavikatty
et al., [13], three patients taken to surgery for management of intussusception demonstrated histologic evidence of florid vascular proliferation involving the resected cecal mass. Given the histologic and clinical features, as well as the lack of recurrence in any of the patients after resection, it was suggested that this vascular abnormality was likely secondary to a reactive process rather than a neoplastic lesion, such as angiosarcoma. Furthermore, whether the lesion preceded the intussusception and served as a lead point, or whether it was a result of continued shear force applied to the bowel wall during intermittent intussusception remained uncertain and difficult to prove [13].Additionally, Adachi
et al., [14] and Ramsden
et al., [15] described acolonic submucosal lipoma serving as a lead point, and histologic evidence of the adjacent and overlaying mucosa consisting of florid vascular proliferations, fibromuscular obliteration, and epithelial regeneration. It was suggested that these unique histologic features demonstrated a reparative process initiated by repeated intussusception or prolapsed mucosa, which subsequently triggered angiogenesis and regenerative changes.
In contrast to these three reports, our case demonstrated a vasculitis, not a vascular proliferation. Our histologic evidence exhibited anatomically normal submucosal vessels that were inflamed with fibrin deposition, not a capillary proliferation of numerous vessels or neovascularization. Additionally, the inflammation was clearly centered on the blood vessels. It is theoretically possible that surgical manipulation and intussusception may cause minimal neutrophilic vascular demargination, but not fibrin deposition. These changes were considered too extensive to be indicative of a reactive process. It is also theoretically feasible for changes suggestive of a vasculitis to be exhibited below an ulcer, but this was not evidenced in our patient.
Other non-neoplastic vascular malformations reported in association with intussusception includearterio-venous malformations, such as angiodysplasia [19]. However, the characteristics of angiodysplasia, as well as other vascular abnormalities of the colon, involve aberrations in the normal vascular architecture, contrasting with our case.
Management of Intussusception in Adults
There are several indications for immediate laparotomy. These include signs of perforation, shock, or peritonitis. In the absence of these signs, the management approach to adult intussusception depends on multiple variables. It is important to consider these variables when making management decisions.
Intussusception of the large bowel requires en bloc resection along with meticulous evaluation of the intra-abdominal cavity in search of metastases because of the higher likelihood of an underlying malignancy as the pathologic lead point.
However, ongoing debate persists regarding the optimal management of adult intussusceptions of the small bowel. Some authors recommend a similar management approach for both the small and large bowel. As suggested by Azar
et al., [2] because approximately 50% of adult colonic and enteric intussusceptions are associated with malignancy, surgical resection is the best treatment option. However, other authors have suggested a more conservative approach involving initial reduction prior to more selective bowel resection. This approach modifies the therapeutic approach to the suspected etiology of the pathologic lead point. Begos
et al., [3] suggested that initial reduction should always be attempted unless there are circumstances involving bowel inflammation, bowel ischemia, bowel friability, and/or colo-colonic intussusception, in which case resection is the optimal management choice.
Manipulation and limited resection of small bowel intussusceptions are controversial because of the inability to reliably distinguish benign and malignant lesions preoperatively by CT evaluation as well as the theoretical risk of disseminating malignant cells. Others have suggested an increased risk of bowel perforation during manipulation of potentially friable bowel as well as the enduring possibility of recurrence that would otherwise be eliminated with en bloc resection [2, 10, 11, 20, 21].
However, those who support the manipulation and limited resection of the small bowel have suggested the avoidance of emergency surgery and the potential prevention of short bowel syndrome, as limited resection could save considerable amounts of bowel. Additionally, because malignant lesions associated with small bowel intussusceptions carry a higher likelihood of metastatic etiology, some argue that manipulation of the bowel would not change the overall prognosis [5]. Limited resection to prevent recurrence is advocated in these cases.
In our case, the diagnosis of intussusception was confirmed by CT and, given the involvement of the colon, the surgical team assumed a malignant pathologic lead point. Therefore, emergency resection of the right colon and the terminal ileum was considered mandatory.
Conclusion
•A vasculitis within the colonic wall serving as the lead point for intussusception is rare in adults.
•Although the definitive etiology of the vasculitis remained unclear, it is important to rule out other non-neoplastic vascular lesions which can occur in the bowel wall. Continued follow-up is also important, because a flare in this disease process could increase the risk of mortality.
•The management of adult intussusception in the large bowel where malignancy is suspected is widely accepted to be surgical and not endoscopic; however, there remains controversy over cases in the small bowel where there is a lower likelihood of malignancy.
Ethical Consideration
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available with the authors.
Competing Interests
We have neither financial nor personal relationships with other people that could inappropriately influence our work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense.
Copyright
The authors of this article are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
Funding
We have no sponsors.
Author Contributions
TO: contributed to the writing of this article as well as in data collection and analysis.
MW: was the first surgeon and contributed to the writing, data analysis and final approval of this article.
TS: contributed to the pathologic analysis of the excised specimen and provided pictures of the pathologic specimen.All authors read and approved the final manuscript for publication.
References
[1].Gayer G, Apter S, Hofmann C, Nass S, Amitai M, Zissin R, Hertz M. Intussusception in adults: CT diagnosis. Clin Radiol 1998;53(1):53-7 [Pubme]
[2].Azar T., Berger D.L. Adult intussusception. Ann Surg. 1997;226(2):134-138 [Pubmed]
[3].Begos DG, Sandor A, Modlin IM. The diagnosis and management of adult intussusception. Am J Surg 1997;73(2):88–94.[Pubmed]
[4].Weilbaecher D, Bolin JA, Hearn D, Ogden W. Intussusception in adults. Review of 160 cases. Am J Surg 1971; 121(5):531-535 [Puibmed]
[5].Barussaud M, Regenet N, Briennon X, de Kerviler B, Pessaux P, Kohneh-Sharhi N, Lehur PA, Hamy A, Leborgne J, le Neel JC, Mirallie E. Clinical spectrum and surgical approach of adult intussusceptions: a multicentric study. IntJ Colorectal Dis 2006;21(8):834-839 [Pubmed]
[6].Felix EL, Cohen MH, Bernstein AD, Schwartz JH. Adult intussusception: case report of recurrent intussusception and review of the literature. Am J Surg 1976;131(6):758–61. [Pubmed]
[7].Takeuchi K, Tsuzuki Y, Ando T, Sekihara M, Hara T, Kori T, Kuwano H. The diagnosis and treatment of adult intussusception. J Clin Gastroenterol 2003;36(1):18-21. [Pubmed]
[8].Erkan N, Haciyanh M, Yildirim M, Sayhan H, Vardar E, Polat AF. Intussusception in adults: an unusual and challenging condition for surgeons. Int J Colorectal Dis 2005;20(5):452-456 [Pubmed]
[9].ubaidi A, Al-Saif F, Silverman R. Adult intussusception: a retrospective review. Dis Colon Rectum 2006;49(10):1546-1551 [Pubmed]
[10].Wang L-T, Wu CC, Yu JC, Hsiao CW, Hsu CC, Jao SW. Clinical entity and treatment strategies for adult intussusceptions: 20 years’ experience. Dis Colon Rectum 2007;50(11):1941-1949 [Pubmed]
[11].Tan KY, Tan SM, Tan AG, Chen CY, Chng HC, Hoe MN. Adult intussusception: experience in Singapore. ANZ J Surg. 2003;73(12):1044-1047 {Pubmed]
[12].Eisen LK, Cunningham JD, Aufses AH. Intussusception in adults: institutional review. J Am Coll Surg. 1999;188:390–395 [Pubmed]
[13].Bavikatty NR, Goldblum JR, Abdul-Karim FW, Nielsen SL, Greenson JK. Florid vascular proliferation of the colon related to intussusception and mucosal prolapse: potential diagnostic confusion with angiosarcoma. Mod Pathol. 2001 Nov;14(11):1114-8 [Pubmed]
[14]Adachi S, Hamano R, Shibata K, Yoshida S, Tateishi H, Kobayashi T, Hanada M. Colonic lipoma with florid vascular proliferation and nodule-aggregating appearance related to repeated intussusception. Pathol Int. 2005 Mar;55(3):160-4.[Pubmed]
[15.]Ramsden KL, Newman J, Moran A. Florid vascular proliferation in repeated intussusception mimicking primary angiomatous lesion. J Clin Pathol. 1993 Jan;46(1):91-2. [Pubme]
[16].Park H, Park S, Hong YJ, Lee SW, Cho MS. Cytomegalovirus-associated intussusception with florid vascuar proliferation in an infant. J Pathol Transl Med. 2015 May;49(3):270-3.[Pubmed]
[17].Fuminori Goda, Takashi Maeba, Hisashi Usuki, Karasawa Y, Izuishi K, Ishimura K, Senda S, Maeta H. Colo-colic intussusception associated with Henoch-Schonlein purpura in adults. J Gastroenterol Hepatol. 2007 Mar;22(3):449-52. [Pubmed]
[18].immerman HJ, Kleitsch WP, Greene AM, Mcfadden HF Jr. Periarteritis (polyarteritis) nodosa producing intussusception; report of two cases. AMA Arch Intern Med. 1954 Aug;94(2):264-71.[Pubmed]
[19].Seddik H, Rabhi M. Two cases of appendiceal intussusception: a rare diagnostic pitfall in colonoscopy. Diagn Ther Endosc. 2011;2011:198984. [Pubmed]
[20].Chang CC, Chen YY, Chen YF, Lin CN, Yen HH, Lou HY. Adult intussusception in Asians: clinical presentations, diagnosis, and treatment. J Gastroenterol Hepatol. 2007;22(11):1767-1771. [Pubmed]
[21].Yamada H, Morita T, Fujita M, Miyasaka Y, Senmaru N, Oshikiri T. Adult intussusception due to enteric neoplasms. Dig Dis Sci 2007;52(3):764-766. [Pubmed]

