Original Article

Expression of vascular endothelial growth factors (VEGF) in head and neck squamous cell carcinoma and adjacent normal tissue
1Devendra Kumar Ravi, 1Vinay Kumar, 2Mohan Kumar, 3Gajendra Singh, 4Shyam Bahadur Rai, 5A. K. Saxena, 1Manoj Pandey
- 1Department of Surgical Oncology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 India
- 2Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 India
- 3Department of Anatomy, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 India
- 4Department of Physics, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 India
- 5Centre for Experimental Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 India
- Submitted: October 15, 2012
- Accepted: November 21, 2012
- Published: November 23, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The prognosis in the head and neck squamous cell carcinoma depends upon the lymphnode status, margin and distant metastasis. Tumor recurrence has been seen inspite of negative margin reported by the pathologist. This has led to the hypothesis that cells have transformed themselves genetically at the molecular level and escape the recognition of the microscopic eye and later phenotypically express as cancer during follow-up.
Angiogenesis is essential for tumor growth and metastasis. Therefore, vascular endothelial growth factor (VEGF) expression increases chances for local relapse, lymph node recurrence and distant metastasis. In this study we have investigated the expression of vascular endothelial growth factor (VEGF) in tumor tissue and adjacent normal tissue and correlated their expression with lymph node metastasis.
Out of 101 patients, 86 (85.1%) patients showed positive expression of VEGF in the tumour tissue. Of these 86, 67 patients showed positive expression for VEGF in the adjacent normal tissue (chi-square = 8.730, p value = 0.0003 with contingency coefficient 0.204). Of the 101 patients, 77 (76.2%) patients were found positive for the cervical lymphnodes. The Pearson correlation coefficient (r) was 0.203 with p value = 0.004 with confidence interval of r = 0.0086-0.3839.
Thus increased expression of VEGF in adjacent normal tissue in the head and squamous cell carcinoma might be the cause of the tumor recurrence or a second primary disease during follow-up and can used as target for the adjuvant therapy in such patients.
Key words
vascular endothelial growth factor (VEGF), surgical margin, local recurrence, head and neck cancer.
Introduction
In oral squamous cell carcinoma poor prognosis is related with loco-regional recurrence and metastatic disease [1,2]. Histological margin reported positive by the pathologist are associated with the local recurrent disease, however failures have been observed in surgical negative margin (30%) due to lack of sensitivity in evaluation of margin (<95%) despite high specificity [3-8]. This has led to identification of cells at the surgical negative margin which have transformed themselves into the cancer cell genetically but has not been expressed phenotypically. Such genetically transformed cells could not be identified in surgical margin by the histologist and later progress to produce recurrent disease. Various studies have investigated the role of genectic alteration at the molecular level, identifying several molecular factors such as TP53, VEGF, MMP9, telomerase activity, microsatellite instability which alter the prognosis in the margin negative cases [9-16].
Angiogenesis is essential for tumor growth and metastasis. Therefore, vascular endothelial growth factor (VEGF) expression increases a chance for local relapse, and lymph node and distant metastasis. High VEGF expressions are associated with the progression of lymph-node spread which are correlated with poor disease free survival [16]. Vascular endothelial growth factor (VEGF) is a signal protein produced by cells that stimulates angiogenesis. VEGF's normal function is to create new blood vessels during embryo development, new blood vessels after injury, muscle following exercise, and new vessels to bypass blocked vessels [17].
Over expression of VEGF contributes to cancers which cannot grow beyond a limited size without an adequate blood supply. 10–20% of dysplastic oral lesions progress to an invasive cancer which is accompanied by increase in blood vessel formation and increased blood supply. Increased expression of VEGF is seen in oral squamous cell carcinoma through all stages of disease progression [18-20]. VEGF has been identified as one of the factors that increases the permeability and it is known to be as vascular permeability factor (VMP) [21-26]. VEGF expression is found in a wide variety of carcinoma including head neck cancer [20,27-32] and its expression is correlated with tumor size, lymph node metastasis and prognosis, however its expression levels in the surrounding tissue and normal tissue is not fully understood. The evaluation of vascular invasion and angiogenesis-associated molecules for the prognosis of Oral Squamous cell carcinoma (OSCC) remains obscure and have been investigated in few studies which is also the case in lymph node metastasis and lymphovasculogenesis-associated molecules [33].
In this study we have evaluated the ability of VEGF expression in identifying tumor-related alterations in histologically negative surgical margins. In this way, we hypothesize that the expression profile of this gene in histologically negative margins could act as a more sensitive and useful marker for the detection of molecular alterations associated with local disease control in HNSCC patients we have inspected in detail the expression of VEGF in the tumor and the peripheral normal tissue in cases with HNSCC and correlated their expression with the metastatic potential of the tumours.
Patients and Methods
This study was carried out in the Department of Surgical Oncology and Department of Pathology, Institute of Medical Sciences Banaras Hindu Varanasi between 2006 - 2011. Tumor specimens from 101 patients Head Neck Squamous cell carcinoma (HNSCC) were collected along with the tissue from the margins. Only patients diagnosed with primary HNSCC, with surgery as primary curative treatment and with surgical margins samples available were included. Histological negative surgical margins were available from all 101 patients in the sample from clinically normal mucosa 1 cm from the tumor and was reported negative by the histopathologist. VEGF protein level was determined by immune histochemistry. The histological grade of tumor was determined and the expression of VEGF was compared with the tumor and those of negative surgical margin findings in all the 101 specimens. The expression of VEGF was also correlated lymph node metastasis in the HNSCC.
4-6 micron thick tissue sections was fixed on clean 1% Poly-L-Lysine coated glass slide and put in incubator at 600C for one hour. All slides were deparaffinize in following solutions for 5 minutes (i). 3x- Xylene, (ii) 2x- 100% ethanol, (iii) 2x- 95% ethanol respectively. After deparaffinization all slides were collected in sodium citrate buffer (pH 6.0) solution and heated at 950C for 10 minute in microwave oven. After cooling, slides were removed from microwave oven and washed with deionized water for one minute then finally fixed in humidified chamber and processed. One drop peroxidase block was added and rinsed with PBS after 2-minute. 1-3 drops of serum block was added and incubated for 20 minutes. Secondary antibody was added after washing with PBS and specimen was incubated for 30 minutes. HRP- Streptavidin complex was also added after washing with PBS and slides were incubated for 30 minutes. HRP Substrate (mixture of deionized water-1.6ml, 5drops 10x substrate buffer, 1-drop 50x DAB chromogen and 1 drop 50x peroxidase substrate) to each slide was added to develop to light brown color in dark humidified chamber about 1-10 minute. Counter stain to slides in Hematoxylin for 5-10 seconds and immediately wash with several changes of deionized water. Slides were destained with acid alcohol and bluing reagent then wash with tap water. Sections were dehydrated as follows: 2x 95% ethanol for 10 second and 2x 100% ethanol for 10 second. Mounting was done with the xylene and DPX. All slides Observed by light microscope and staining was graded as follows:- (i). Positive, (ii). Negative, (iii). Equivocal. Beside the following were also recorded for each slide; type of staining :- (i). Cytoplasmic (ii) Nuclear (iii) Membranous
The association between the clinical variables and molecular data was evaluated using Chi-square test. The association of the molecular markers and clinical variables like lymph node metastasis were calculated using the Pearson correlation coefficient. All statistical analysis was performed using the statistical software of MEDCALC and a level of significance of p
< 0.05 was taken as standard.
Result
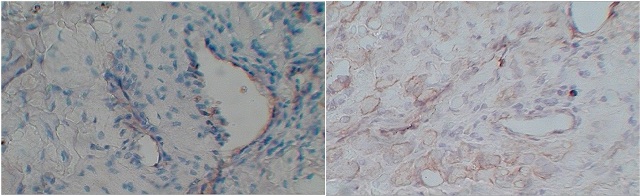
Figure 1: A. Photomicrograph showing negative staining H&E X100 B). Cytoplasmic 1+ expression of VEGF in SCC grade 1.
Majority (43.5%) patients were of carcinoma buccal mucosa, 11.9% patients had lesions on tongue, 18.8% patients had carcinoma alveolus and 25.7% of patients lip and gingiobuccal sulcus. Of these, 41.5% tumors were well differentiated, 31.6% moderately differentiated and 26.7% were poorly differentiated. Out of 101 patients, 86 (85.1%) patients showed positive expression of VEGF in the tumour tissue (figure 1). Similarly 67 (66.3%) patients showed positive expression for VEGF in the adjacent histologically negative normal tissue (chi-square = 8.730, p value = 0.0003 with contingency coefficient 0.204). Of the 101 patients, 77 (76.2%) patients were found positive for the cervical lymphnodes. The degree of association between VEGF expression and lymph node metastasis was analysed using Pearson correlation coefficient (r) was 0.203 with p value = 0.004 with confidence interval of r = 0.0086-0.3839.
Discussion
It has been supposed that tumour cells undergoing genetic alteration in surgical margins reported negative by the histologist are responsible for tumour recurrence and prognosis of the patient in head neck squamous cell carcinoma. In this study we have demonstrated the over expression of VEGF in the tumor cell and adjacent negative margin in the surgically treated case of oral squamous cell carcinoma and its correlation with lymph node metastasis. The VEGF over expression (in the tumor cell) has been significantly correlated (p=0.004) with the lymphnode metastasis. 76.2% of the patients has been positive for cervical lymph nodes .66.3% patients in adjacent tissue reported normal were also investigated for the higher expression of VEGF. These are the cells which are probably responsible for the local or lymph node recurrence. Many other studies have reported high VEGF expression associated with the progression of lymph node spread (p= 0.0009), which are correlated with poor disease free survival (DFS) [4], where 2-year DFS rate of high VEGF expressors (30%) was significantly lower than that of low VEGF expressors (78%) (p= 0.0008). It has shown that increased expression of this gene leading to enhanced synthesis of this protein (VEGF), independently on other factors, increases a chance for local relapse, and distant metastasis [34]. Consequently, patients with oral cancer have poor disease-free survival, as well as poor overall survival. In a similar study the results showed that 11 (17.5%) patients had VEGF expression less than 20% and 29 (82.5%) above 20% [34]. A statistical significance was immanent with positive nodal status (p
< 0.05) and disease stage (p < 0.05). No statistical correlation was found between the level of VEGF expression and histological and nuclear grade, tumor size, disease relapse or patients overall survival.
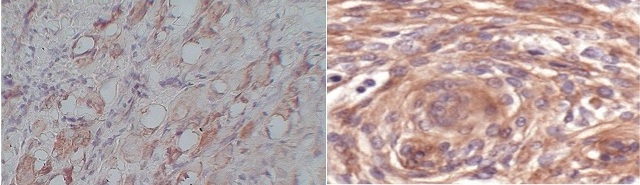
Figure 2: Moderate (2+) cytoplasmic cepression of VEGF in grade II SCC H&E X 100 B). Strong cytoplasmic expression of VEGF in grade III SCC. H & E X400.
Our results of 85.1% expression of VEGF in tumor tissue are comparable to the literature and investigation of positive expression of VEGF (66.3%) in adjacent normal tissue throws new light in explaining the cause of the recurrence of oral cancer even when reported negative by the pathologist. Thus VEGF expression in adjacent normal tissue may play an important role in progression of HNSCC. Many other researchers have also demonstrated that demonstrated that the overexpression of tumor-related genes in histologically negative surgical margins is a frequent event by the use of quantitative RT–PCR may be an useful tool in detecting actually negative HNSCC surgical margins and the overexpression of specific genes in these margins could be helpful in the identification of patients with a higher risk of developing second primary tumor and local recurrences [35].
Based on these results, we may speculate that the expression of these genes in HNSCC surgical margins, diagnosed as tumor-free by conventional histopathology, could be a helpful biomarker to identify subjects at risk of new disease. It can also be used as an alternative tool to guide the surgeon in the delineation of the HNSCC resection extent and also during follow-up in the planning of adjuvant therapy.
Authors' Contribution
DKR: Conducted the study, did the literature search and prepared the manuscript
VK: Helped in literature search and preparation of manuscript
MK: Conceived and designed the pathological part of the experiment and contributed to the manuscript
GS: Contributed to the electron microscopy and edited the final manuscript.
SBR: Conceived and designed the LASER experiment and contributed to preparation of manuscript.
AKS: Preparation and revision of the manuscript.
MP: Conceived and designed the study and edited the final manuscript for publication
All authors have seen the final manuscript and approve its publication.
Conflict of Interests
The authors declare that there are no conflict of interests
Ethical Consideration
The study was approved by the Ethics committee of the Institute of Medical Sciences
Acknowledgement
DKR was supported by the Rajiv Gandhi National Fellowship of the University Grants Commission.
Funding
None
References
[1]. Katherine Holmes, Owain LI Roberts, Angharad M. Thomas, Michael J Cross. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signaling and therapeutic inhibition. Cellular Signaling 2007; 19 (10):2003–12. [Pubmed].
[2]. Johnstone S, Logan RM. Expression of vascular endothelial growth factor (VEGF) in normal oral mucosa, oral dysplasia and oral squamous cell carcinoma. International journal of oral and maxillofacial surgery. 2007;36(3):263–66. [Pubmed].
[3]. Bowden J, Brennan PA, Umar T, Cronin A. Expression of vascular endothelial growth factor in basal cell carcinoma and cutaneous squamous cell carcinoma of the head and neck. Journal of cutaneous pathology. 2002; 29(10):585–589. [Pubmed].
[4]. Mineta H, Miura K, Ogino T, et al. Prognostic value of vascular endothelial growth factor (VEGF) in head and neck squamous cell carcinomas. British journal of cancer. 2000;83(6):775–781. [Pubmed].
[5]. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983. 219: 983–985. [Pubmed].
[6]. Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989. 161: 851–858. [Pubmed].
[7]. Ferrara N, Leung DW, Cachianes G, Winer J, Henzel WJ. Purification and cloning of vascular endothelial growth factor secreted by pituitary folliculostellate cells. Methods Enzymol 1991; 198: 391–405. [Pubmed].
[8]. Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem 1991; 47: 211–218. [Pubmed].
[9]. Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biologicalproperties of the vascular endothelial factor family of protein. Endocr Rev 1992; 13: 18–32. [Pubmed].
[10]. Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989; 246: 1309–1312. [Pubmed].
[11]. Eisma RJ, Spiro JD, Kreutzer DL .Vascular endothelial growth factor expression in head and neck squamous cell carcinoma. Am J Surg 1997; 174: 513–517. [Pubmed].
[12]. Sauter ER, Nesbit M, Watson JC, Klein-Szanto A, Litwin S, Herlyn M. Vascular endothelial growth factor is a marker of tumor invasion and metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res 1999;5: 775–782. [Pubmed].
[13]. Takano S, Yoshii Y, Kondo S, Suzuki H, Maruno T, Shirai S, Nose T. Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res 56: 2185–2190. [Pubmed].
[14]. Fontanini G, Boldrini L, Chine S, Pisaturo F, Basolo F, Calcinai A, Lucchi M, MussiA, Angeletti CA, Bevilacqua G. Expression of vascular endothelial growth factor mRNA in non-small cell lung carcinomas. Br J Cancer 1999; 79: 363–369. [Pubmed].
[15]. Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, Ellis LM. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg 1997; 132: 541–546. [Pubmed].
[16]. Kumar H, Heer K, Lee PWR, Duthie GS, MacDonald AW, Greenman J, Kerin MJ, Monson JR. Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer. Clin Cancer Res 1998; 4: 1279–1285. [Pubmed].
[17]. Hinerman RW, Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35 year experience. Head Neck 2004;26(11):984–94. [Pubmed].
[18]. Leemans CR, Tiwari R, Nauta JJ, Van dW I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph nodemetastases as a prognostic factor. Cancer 1994;73(1):187–90. [Pubmed].
[19]. Ikemura K, Ohya R. The accuracy and usefulness of frozen-section diagnosis.Head Neck 1990;12(4):298–302. [Pubmed].
[20]. Gandour-Edwards RF, Donald PJ, Wiese DA. Accuracy of intraoperative frozen section diagnosis in head and neck surgery: experience at a university medical center. Head Neck 1993;15(1):33–8. [Pubmed].
[21]. Gandour-Edwards RF, Donald PJ, Lie JT. Clinical utility of intraoperative frozen section diagnosis in head and neck surgery: a quality assurance perspective.Head Neck 1993;15(5):373–6. [Pubmed].
[22]. Spiro RH, Guillamondegui O Jr, Paulino AF, Huvos AG. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck 1999;21(5):408–13. [Pubmed].
[23]. Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys 1997;39(1):137–48. [Pubmed].
[24]. Laccourreye O, Hans S, Menard M, Garcia D, Brasnu D, Holsinger FC. Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region: II. An analysis of the incidence, related variables, and consequences of local recurrence. Arch Otolaryngol Head Neck Surg. 2005;131(7):592-9. [Pubmed].
[25]. van Houten VM, Leemans CR, Kummer JA, Dijkstra J, Kuik DJ, van den Brekel MW, et al. Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients: a prospective study. Clin Cancer Res 2004;10(11): 3614–20. [Pubmed].
[26]. Poeta ML, Manola J, Goldenberg D, Forastiere A, Califano JA, Ridge JA, et al. The Ligamp TP53 assay for detection of minimal residual disease in head and neck squamous cell carcinoma surgical margins. Clin Cancer Res 2009;15(24): 7658–65. [Pubmed].
[27]. Nathan CO, Franklin S, Abreo FW, Nassar R, de Benedetti A, Glass J. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J Clin Oncol 1999;17(9):2909–14. [Pubmed].
[28]. Nathan CO, Amirghahri N, Rice C, Abreo FW, Shi R, Stucker FJ. Molecular analysis of surgical margins in head and neck squamous cell carcinoma patients. Laryngoscope 2002;112(12):2129–40. [Pubmed].
[29]. Goldenberg D, Harden S, Masayesva BG, Ha P, Benoit N, Westra WH, et al. Intraoperative molecular margin analysis in head and neck cancer. Arch Otolaryngol Head Neck Surg 2004;130(1):39–44. [Pubmed].
[30]. Martone T, Gillio-Tos A, De ML, Fiano V, Maule M, Cavalot A, et al. Association between hypermethylated tumor and paired surgical margins in head and neck squamous cell carcinomas. Clin Cancer Res 2007;13(17):5089–94. [Pubmed].
[31]. Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch WM, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med 1995;332(7):429–35. [Pubmed].
[32]. H Mineta, K Miura, T Ogino, S Takebayashi,K Misawa, Y Ueda, I Suzuki, M Dictor,Å Borg, J Wennerberg. Prognostic value of vascular endothelial growth factor (VEGF) in head and neck squamous cell carcinomas.Br J Cancer. 2000; 83(6): 775–781. [Pubmed].
[33]. Seki S, Fujiwara M, Matsuura M, Fujita S, Ikeda H, Asahina I, Ikeda T. Prediction of outcome of patients with oral squamous cell carcinoma using vascular invasion and the strongly positive expression of vascular endothelial growth factors. Oral Oncol 2011 July;47(7): 588-93. [Pubmed].
[34]. Broćić M, Kozomara R, Cerović S, Jović N, Vukelić-Marković S, Stosić S. Clinical significance of vascular endothelial growth factor expression in patients with carcinoma of the mouth floor and tongue. Vojnosanit Pregl. 2009 Jun;66(6):440-8. [Pubmed].
[35]. De Carvalho AC, Kowalski LP, Campos AH, Soares FA, Carvalho AL, Vettore AL. Clinical significance of molecular alterations in histologically negative surgical margins of head and neck cancer patients. Oral Oncology 48 (2012) 240–248. [Pubmed].

