Case Report

Metastatic follicular carcinoma of thyroid mimicking primary bone tumor
1Krishnakiran K, 1Vineeta Srivastava, 2Hema Malini Iyer, 1Manoj Pandey
- 1Department of Surgical Oncology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221 005, India
- 2Department of Pathology, Lal Pathology, New Delhi, India
- Submitted: November 15, 2011;
- Accepted December 30, 2011
- Published: January 10, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction:
Follicular carcinoma of the thyroid metastasize to bones very commonly, however, its presentation as a long standing soft tissue mass is rare.
Case presentation:
A 74 year old lady presented with slowly progressive swelling in the gluteal region for 10 years with sudden increase in size for last two months. Biopsy showed it to be a metastasis from follicular thyroid carcinoma. Search for the primary revealed two occult nodule in thyroid. Patient was treated with total thyroidectomy followed by radio iodine ablation.
Conclusion:
Presentation of thyroid carcinoma as primary bone and soft tissue tumor is rare and mimic primary bone tumor or soft tissue sarcoma. Treatment is by Total thyroidectomy followed by radio iodine ablation.
Introduction
Differentiated thyroid carcinoma (DTC) presenting with metastatic disease and occult primary is rare. Though, bone metastasis develops in 7-28% of follicular thyroid cancers (FTC) [1], and at the time of initial presentation approximately 11% of all patients with FTC have bone metastasis [2]. In majority of these patients, goiter is present in addition to the symptoms and signs related to the bone metastasis. Survival in patients with bone metastasis at presentation is estimated to be 39% at 5 years, 31% at 10 years and 23% at 15 years [3]. For patients without bone metastasis, the 10 year survival is 80-95% [1]. We report here an unusual case of a 74 year old female who presented to us with a 10 year history of bone metastasis from follicular thyroid cancer in the absence of a goiter, mimicking a primary bone tumor.
Case report
A seventy four year old female presented with complaint of a slow growing swelling in the left gluteal region for ten years duration. There was a rapid increase in size of the swelling over the last two months. The lesion was painless to start with but since two months the patient is complaining of increasing pain requiring the use of analgesics. There was no history of trauma, or fever associated with the lesion. The patient has no difficulty in walking or squatting. She was otherwise healthy and had no symptoms pertaining to the central nervous, cardiovascular, respiratory, gastrointestinal or genitourinary systems. Her past medical history was not contributory. She had undergone surgery for cataract in her right eye 2 years ago followed by loss of vision in the eye. Her ECOG performance status was zero, general examination was unremarkable. She had no goiter on examination of the neck (Figure 1). Patient was clinically euthyroid. Her resting pulse rate was 70bpm and BP 160/100 mm of Hg.
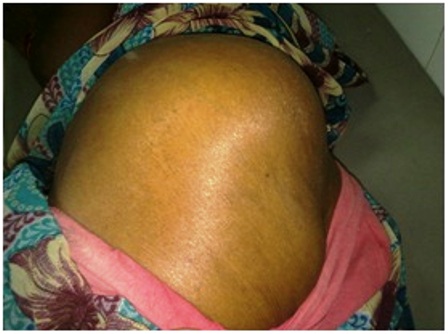
Figure 1- lesion in the left gluteal region
Examination of the left gluteal region revealed a 12 x 12 centimeter swelling extending from the anterior superior iliac spine to the sacrum posteriorly and from the iliac crest to the ischial tuberosity inferiorly (Figure 2). The lesion had diffuse borders and a variable consistency with solid and cystic areas. The lesion was pulsatile and was fixed to the iliac bone. The overlying skin was stretched and shiny with dilated surface veins. On digital rectal examination, a fixed extramural mass was felt on the left lateral rectal wall pushing the rectum to the right. The patient had no other palpable lump.
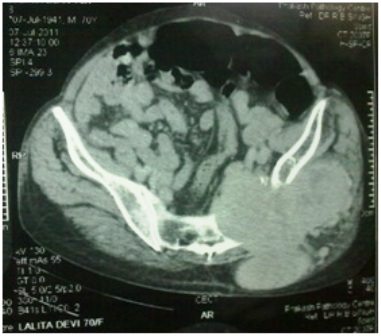
Figure 2- CT scan showing the lesion arising from left iliac bone
A clinical diagnosis of primary bone tumor or bone secondaries was made.
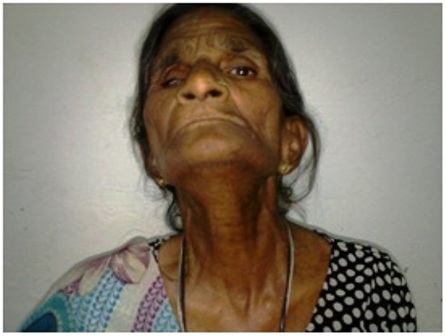
Figure 3- photograph of the patient showing absence of a goiter
Contrast enhanced CT scan of the pelvis showed a 11x12x11 cm osteolytic lesion involving ilium and sacrum in the region of the left sacroiliac joint with intrapelvic and extrapelvic extension (Figure 3). The lesion involved the iliacus muscle, gluteus maximus and medius muscles with extension into the subcutaneous plane in the gluteal region and was surrounded at the periphery by multiple dilated tortuous vessels. A trucut biopsy of the mass was obtained that revealed follicular structures containing eosinophilic colloid material resembling thyroid parenchyma (Figure 4). Immuno histochemistry for thyroglobulin was done. The follicular cells had an immunoreactive score of 3 (Figure 5).
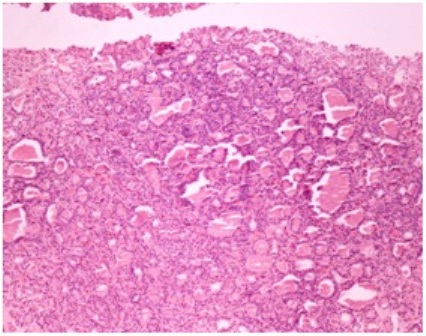
Figure 4- Histopathology of core needle biopsy showing follicular structures with eosinophilic colloid material resembling thyroid parenchyma
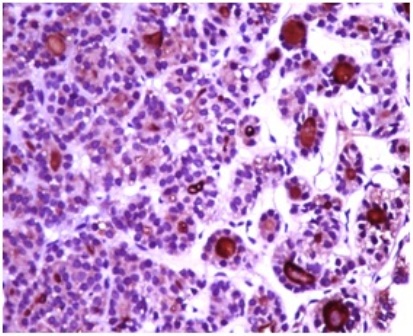
Figure – 5 photomicrograph showing strong Immunoreactivity for thyroglobulin
With a diagnosis of bone metastasis from follicular carcinoma of the thyroid, Ultrasound of the neck was done to look for subclinical thyroid nodules. Multiple sub centimeter nodules were found in both lobes of the thyroid. There was no cervical lymphadenopathy. Her thyroid profile was normal. Patient underwent total thyroidectomy after control of hypertension. The final histopathology revealed follicular carcinoma of the thyroid. Patient was treated with postoperative radioiodine ablation.
Discussion
Differentiated thyroid cancer (DTC) rarely has metastatic disease at presentation. In a study at Memorial Sloan-Kettering Cancer center, 4% of DTC patients had distant metastases at presentation, 2.3% in papillary thyroid cancer (PTC) and 11% in follicular thyroid cancer (FTC)[2]. In this study, long term survival was 43% in patients with distant metastasis, when compared to 86% in patients without distant metastasis. Bone metastasis are seen in 7-28% of FTC patients and 1.4-7% of PTC patients [1]. Survival in patients with bone metastasis at presentation is estimated to be 39% at 5 years, 31% at 10years and 23% at 15 years [3]. For patients without bone metastasis, the 10 year survival ranges from 80-95% [1]. Lung is the most common site of distant metastasis followed by bone [1]. Symptoms and signs of patients presenting with bone metastasis depend on the site involved. Most common symptom is pain which becomes severe over time. Other presentations are fracture and spinal cord compression. Bone metastasis from DTC are osteolytic [1]. 80% are located in the axial skeleton (vertebra, ribs and hips). Plain x-rays are not sensitive for diagnosis as lesions less than 1cm are not detected. CT scan has a sensitivity of 70-100 % [1]. MRI has a sensitivity of 94% and accuracy of 91 % [1]. Bone scan (Tc99m MDP) has high false positive and false negative rates [1]. I123 and I131 radioiodine scans are the mainstay of diagnosis. Recently, functional imaging has gained importance in detecting bone metastases from DTC. FDG-PET is useful in metastatic poorly differentiated thyroid cancers which do not take up I131. I124 PET-CT was found in a recent study to detect 100% of lesions, when compared to I131 whole body scan which detected 83% of lesions, and CT scan which detected 56% of lesions [4].
In several studies, the prognosis of patients with bone metastasis was found to be dependant mainly on the age of the patient at presentation, number of metastatic sites and response to radioiodine therapy [3]. Radioiodine ablation remains the mainstay of treatment for bone metastasis from DTC. Before radioiodine ablation of metastasis, patients undergo total thyroidectomy in order to facilitate radioiodine uptake by the metastatic sites. Bone metastasis are in general resistant to treat with curative intent when compared to lung metastases. A study by Zhong-Ling Qiu et al. reported on the efficacy of I131 ablation for bone metastasis from DTC [5]. Response was evaluated by change in serum thyroglobulin (Tg) levels, palliation of bone pain and imaging changes. 34.9% patients had decrease in serum Tg levels, 63.9% o patients had significant relief of bone pain. However, 76% of patients had no anatomical imaging changes on imaging after I131 ablation. In another study Casara et al., [6], 214 patients with metastatic DTC were studied after treatment with radioiodine. In this study it was found that radioiodine uptake, age at presentation, site of metastases, and numbers of metastases were associated with prognosis after I131 ablation. Patients who had no radioiodine uptake by the metastases were never disease free after treatment. Patients with bone metastasis had the worst prognosis. Tumor histology also influenced survival with FTC having poorer prognosis than PTC.
Treatment options for patients with radioiodine resistant tumors include surgery for lesions amenable to excision, non surgical ablative techniques such as radiofrequency ablation and percutaneous ethanol injection [7] and external beam radiation therapy (EBRT)[1]. EBRT is an effective palliation for bone metastasis from DTC with 80% of patients having pain relief [1], with single fraction regimens being equally effective and more convenient than conventional schedules [8]. Recent NCCN guidelines recommend the use of small molecule kinase inhibitors sunitinib and sorafenib for non CNS metastatic sites which are refractory to radioiodine ablation
[9].
Author Contributions
KKK did the literature search and prepared the draft manuscript.
VS helped in literature search and preparation of the manuscript.
HMI Contributed to the pathology part of the manuscript and provided the photomicrography images.
MP: Designed the study and edited the final manuscript.
Conflict of interests
The authors declare that there are no conflicts of interests
Patient consent
Written consent was obtained from the patient for reporting of this case
Funding Source
Nil
References
[1]. Muresan MM, Olivier P, Lecle`re J, Sirveaux F, Brunaud L, Klein M, Zarnegar R, Weryha G. Bone metastases from differentiated thyroid carcinoma Endocrine-Related Cancer 2008; 15: 37–49
[2]. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg 1997;174: 474-476
[3]. Schlumberger M, Tubiana M, De Vathaire F, Hill C,Gardet P, Travagli JP, Fragu P, Lumbroso J, Caillou B, Parmentier C. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metabolism 1986; 63: 960–967.
[4]. Freudenberg LS, Antoch G, Jentzen W, Pink R, Knust J, Gorges R, Muller SP, Bockisch A, Debatin JF, Brandau W. Value of (124)I-PET/CT in staging of patients with differentiated thyroid cancer. European Radiology 2004; 14: 2092-2098
[5]. Qiu ZL, Song HJ, Xu YH, Luo QY. Efficacy and Survival Analysis of 131I Therapy for Bone Metastases from Differentiated Thyroid Cancer
J Clin Endocrinol Metabolism 2011 July 27
[6]. Casara D, Rubello D, Saladini G, Gallo V, Masarotto G, Busnardo B. Distant metastases in differentiated thyroid cancer: long-term results of radioiodine treatment and statistical analysis of prognostic factors in 214 patients. Tumori 77: 432-436.
[7]. Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg 244: 296-304
[8]. Falkmer U, Jarhult J, Wersall P, Cavallin-Stahl E. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncologica 2003;42: 620–633.
[9]. National Comprehensive Cancer Network: NCCN guidelines: thyroid cancer treatment guidelines. 2011:25 [available at http://www.nccn.org; last accessed December 20, 2011]

