Research

Expression of Müllerian Inhibiting Substance (MIS) and its receptor in female genital tract
1Ham Bak Lee, 2Yoon Ji Jung, 1Min Jung Kim, 2Hyun Hee Cho, 2Mee Ran Kim, 1Eun Jung Kim, 2Jang Heub Kim
- 1Department of obstetrics and gynecology, Bucheon St. Mary's Hospital, The Catholic University, South Korea
- 2Department of obstetrics and gynecology, Seoul St. Mary's Hospital, The Catholic University, Seoul, South Korea
- Submitted: December 18, 2012;
- Accepted: January 06, 2013,
- Published: February 01, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objective
Müllerian inhibiting Substance (MIS), also known as anti Müllerian hormone (AMH), is produced from Sertoli cells of fetal testis and it causes regression of Müllerian ducts. MIS is known to act as a regulator of female reproductive function but also inhibits the growth of Müllerian duct-derived tumors in vivo and in vitro. But the physiologic role of MIS in female genital tract is not known clearly. Therefore, this study is aimed to confirm the expression of MIS and MISRII in female genital tract.
Material and Methods
We gathered the tissues of female genital tract from the patients, who had the hysterectomy for benign uterine diseases like myoma uteri or adenomyosis. We divided the patients into follicular phase and luteal phase by menstrual cycle. We had undergone our experiment by using tissues from the ovary, uterine cervix, uterine endometrium, uterine myometrium and tube for each patient. We performed immune histochemistry and RT-PCR to confirm MIS and MISRII in each tissues of female genital tract.
Results
MIS was only expressed in ovarian tissue among the tissues of female genital tract. And MISRII was expressed in the entire female genital tract. But MISRII's expression intensity was different relatively. MISRII was very strongly expressed in ovary, and moderately expressed in uterine cervix, uterine endometrium, and fallopian tube. But MISRII was only mildly expressed in uterine myometrium with RT-PCR.
Conclusion
Finally we found that MIS is expressed only in ovary of female genital tract. And MISRII is presented on the entire female genital tract but the expression intensity was different relatively. These results suggest that MIS may have the function of biological modifier or inhibitor on female genital organ development and maturation. We could use this study for basic useful research to understand the physiology of MIS.
Key words
Müllerian inhibiting substance; MIS type II receptor; Immunohistochemistry; RT-PCR; in situ hybridization.
Background
In 1946, Alfred Jost found mullerian duct degeneration when he removed the undifferentiated gonad and inserted the testis in rabbit embryos. He named the substance secreted from the testis Müllerian inhibiting substance (MIS) [1]. Bioassay on MIS was developed in 1969 [2], and MIS was purified from cows [3]. Enzyme-linked immunosorbent assay on MIS was conducted using a monoclonal antibody in 1983 [4]. In 1990, MIS was quantified using purified recombinant human MIS (rhMIS) via ELISA [5].
MIS or Anti Müllerian hormone (AMH) is produced in immature Sertoli cells of the fetal testis, and then binds to its receptors present in the mesenchymal cells nearby mullerian duct, thereby degenerating mullerian duct. Via this mechanism, it plays an important role in sex development by inhibiting the development of the fallopian tube, uterine, and upper vagina in men. In addition, it is also involved in fatal lung maturation and testis differentiation and descending. In women, MIS is rarely produced in women aged less than 10 years. After adolescence, it is produced in ovarian granulosa cells, and secreted into the serum and follicular fluid at a similar concentration as that of men. Thus, it plays as a factor inhibiting follicle selection, oocyte maturation, and steroid production in fertile women via autocrine, paracrine, and endocrine [6, 7].
MIS receptor is a heterometric complex that consists of tissue-specific type I and II transmembrane serine threonine kinase receptor. MISRII is a 63 kDa protein consisting of 11 exons. The 25 kDa C-terminus, which is a proteolysed part of MIS, binds to MISRII that specifically binds to ligand, thereby inducing signal transduction via MISRI phosphorylation [8, 9]. Four cellular signal transduction pathways have been mainly identified. They include Smad pathway, β-catenin/lymphoid enhancer factor-1 (LEF-1) pathway, Cyclin-dependent kinase inhibitor (CDKI) pathway, and nuclear factor кB (NFкB) pathway [10, 11].
MIS inhibits the development of the fallopian tube, uterine, and upper vagina in men by degenerating mullerian duct. In addition, Leydig cell tumor and hyperandrogenism occur in MIS-knock-out male mice, and the stimulated follicles are early lost in female mice [12-14]. Furthermore, considering that MIS is found only in fertile women, MIS is expected to be involved in the mechanism of reproductive endocrinology, associated with ovarian development. However, no definite menstrual mechanism has been identified yet. Although studies on MIS receptor were required to elucidate MIS mechanism and its role in menstruation, few studies have been conducted to date. In addition, current studies on MIS and MISRII have been mainly focused on the ovary among female reproductive organs.
Accordingly, this study was conducted to investigate the expression of MIS and MISRII in five female reproductive organs (ovary, uterine cervix, uterine endometrium, uterine myometrium, fallopian tube) and to explore its clinical application.
Material and Methods
Subjects
This study was conducted on women with regular menstruation cycle among fertile women aged 30~45 years who underwent hysterectomy due to benign gynecologic disease from March 2011 to August 2011. Immunohistochemistry and RT-PCR were conducted on the tissues collected from the ovary, uterine cervix, uterine endometrium, uterine myometrium, and fallopian tube in order to exam the expression of MIS and MISRII.
10 subjects were divided into two groups according to menstruation cycle: follicular phase and luteal phase. The subject’s age ranged from 37 years to 45 years (mean age: 42.6 years), and their menstruation cycle was 27-31days. RT-PCR was performed on two subjects at follicular phase and another two subjects at luteal phase. This study was conducted after obtaining the informed consent form and the approval from the clinical study center of catholic medical center in accordance with “Bioethics and Security Act” (XC11TASS0061) (Table 1).
Table 1: Patients characteristics and findings
| Patients in follicular phase |
|
|
|
|
|
| Age (years) |
45 |
45 |
44 |
44 |
43 |
| Parity |
1-0-1-1 |
2-0-1-2 |
1-0-0-1 |
2-0-1-2 |
2-0-0-2 |
| Height (cm) |
155 |
160 |
156 |
162 |
166 |
| Weight (kg) |
65 |
58 |
53 |
51 |
59 |
| BMI (kg/m2) |
27 |
22.6 |
21.8 |
19.4 |
21.4 |
| Operation history |
C/S* |
No |
No |
No |
C/S |
| MCD** on operation |
8 |
3 |
12 |
11 |
2 |
| Menstrual history |
|
|
|
|
|
| Regularity |
regular |
regular |
regular |
regular |
regular |
| Cycle (days) |
28 |
30 |
31 |
28 |
30 |
| Duration (days) |
7 |
5-7 |
5 |
4 |
7 |
| Amount |
profuse |
moderate |
scanty |
moderate |
profuse |
| Dysmenorrhea |
severe |
no |
no |
no |
severe |
| Pathology |
myoma |
myoma |
myoma |
adenomvosis |
adenomvosis |
| Patients in luteal phase |
|
|
|
|
|
| Age (years) |
41 |
39 |
37 |
43 |
45 |
| Parity |
1-0-0-1 |
2-0-0-2 |
2-0-1-2 |
2-0-1-2 |
2-0-3-2 |
| Height (cm) |
168 |
160 |
158 |
162 |
156 |
| Weight (kg) |
70 |
65 |
59 |
65 |
68 |
| BMI (kg/m2) |
24.8 |
25.4 |
23.6 |
24.8 |
28 |
| Operation history |
No |
C/S |
myomectomy |
C/S |
No |
| MCD** on operation |
28 |
22 |
25 |
24 |
27 |
| Menstrual history |
|
|
|
|
|
| Regularity |
regular |
regular |
regular |
regular |
regular |
| Cycle (days) |
27 |
28 |
30 |
29 |
28 |
| Duration (days) |
7 |
7 |
10 |
7 |
5 |
| Amount |
profuse |
moderate |
profuse |
moderate |
moderate |
| Dysmenorrhea |
no |
severe |
no |
no |
moderate |
| Pathology |
myoma |
adenomyosis |
myoma |
myoma |
myoma |
*C/S: cesarean section
** MCD: menstrual cycle day
Sampling
After surgery, approximately 1cm3 – sized tissue was collected from five organs (ovary, uterine cervix, uterine endometrium, uterine myometrium, and fallopian tube) of each subject, and put into neutral formalin. The sample tissues were put into 10% neutral formalin, followed by fixation for more than 24 hours. After washing the sample tissues using flowing water, the samples were sequentially dehydrated with 70, 80, 90, and 100% ethanol using Tissue Tek VIP5 automated processor (Sakura Finetek, Torrance, CA). After vitrification, they were embedded on paraffin using an embedding apparatus (Histocentre 2, Shandon, USA), and sliced to prepare tissue sections in 4 µm thickness using a thin sectioner (MICROM HM 400R, Walldorf, Germany). Then, the prepared tissue sections were placed on slide glass (Probe on slide, Fisher Scientific Co., Pittsburgh, PA), followed by Immunohistochemistry. The fresh tissues were sRNA, followed by RT-PCR.
Immunohistochemistry
MIS expression
For deparaffining and hydration of the prepared specimen, the specimen was reacted with xylene for 15min, and then reacted with 100%, 95%, 90%, 80%, and 70% alcohol in that order for 5 min, respectively. To expose to antigen, the specimen was put into 1x sodium citrate buffer (Zymed Lab. Inc., San Francisco, CA), followed by pretreatment in a microwave oven for 15 min. After washing with PBS (pH 7.4) three times for 10 min each, the specimen underwent peroxidase block for 10 min, and washing again. The specimen was put into 10% normal donkey serum (Jackson immunoresearch Laboratories, lnc, USA) at RT for 1 hr to remove non-specific reactions. Then, the specimen was treated with a primary antibody, MGH6 (provided by Professor David MacLaughlin, Massachusetts General Hospital, and Boston, MA) at 4°C for 24 hr, followed by washing with PBS three times for 5 min each. Next day, after washing with PBS three times for 10 min each, the specimen was treated with a secondary antibody, biotinylated antibody (Histostain-Plus, Zymed) at RT for 1 hr. The specimen was washed with PBS again, and then treated with HRP-cojugated-streptavdin for 30 min. The specimen was washed with Tris buffer (TB, 0.05 M, pH 7.6) three times for 10 min each, and then treated with 3-amino-9-ethylcarbazole (AEC, Lab vision) at RT for 10 min for color reaction, followed by control-staining with hematoxylin. After immunostaining, the slide was embedded on glycerol gel, followed by observation.
Expression of MIS type II receptor
Immunohistochemistry of MIS type II receptor was conducted using anti-human MIS type II receptor multi-clonal antibody (provided by professor, David MacLaughlin) that was prepared from rabbits using the primary antibody in a same way as that of MIS.
Reverse transcriptional polymerase chain reaction (RT-PCR)
RNA extraction and reverse transcription
RNA was extracted from a total of 20 sample tissues from the five organs (ovary, uterine cervix, uterine endometrium, uterine myometrium, and fallopian tube) in four patients, 2 patients for follicular phase and luteal phase, respectively. The total RNA was extracted using TRIZOL LS Reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was conducted on the extracted RNA using cDNS Synthesis Kit (Takara Korea Biomedical Inc., Korea) for cDNA production. That is, a total of 20 ㎕ reaction solution including 5x reverse transcription buffer 4 ㎕, MMLV reverse transcriptase 1 ㎕, dNTP mix 1 ㎕, random primer 2 ㎕, RNase inhibitor 1 ㎕, RNA 2 ㎍, and DEPC-treated deionized water was prepared, and then reacted in a DNA thermal cycler at reaction conditions of 25 °C, 10min; 42 °C, 60min; and 70 °C, 10 min. After reaction, the product was stored at -20 °C.
RT-PCR
PCR reaction was performed using Premix Taq (Takara Ex Taq version) (Takara Korea Biomedical Inc., Korea). PCR premix 10 ㎕, downstream primer (10 pmol) 1 ㎕, upstream primer (10 pmol) 1 ㎕, reverse transcriptional cDNA 1 ㎕ and RNase free sterile water were mixed to prepare 20 ㎕ reaction solution, followed by PCR at reaction conditions of 94 °C, 5min for early denaturation; 94 °C, 40sec, 57 °C, 40sec, 72 °C, 25sec for 30-cycle extension;, and 72 °C, 10 min for final reaction. Some portion of human MISRII cDNA sequence (Gene Bank, Accession No: AF172932) was used for the primer sequences used. The 20-nucleotide sequence between 581 and 600 (5’ccctgctacagcgaaagaac 3’) was selected for sense primer, whereas the 20-nucleotide sequence between 921 and 941(5’ tgggtcaagtagtggcacag 3’) was selected for antisense primer. After PCR, 10 ㎕ of PCR product underwent electrophoresis using 1.5% agarose gel, followed by the identification of 361 bp bands.
Result interpretation
The result of Immunohistochemistry was assessed to be no staining, weak, intermediate, and strong staining by two histopathologists according to staining level.
Result
MIS expression via Immunohistochemistry
In the ovary, a strong MIS expression was observed in preantral and antral follicles at the follicular phase, but MIS was detected only in granulosa cells (Figure 1A, 1B). Meanwhile, the MIS expression was observed only in a small number of cells in the Corpora Luteum at lueal phase, nd no MIS expression was shown in the egg, ovary stroma, atretic follicles, epithelium and corpus albicans. Furthermore, no MIS expression was observed in the uterine cervix (Figure 1C), uterine endometrium (Figure 1D), uterine myometrium (Figure 1E), and fallopian tube (Figure 1F) for both follicular phase and luteal phase groups.
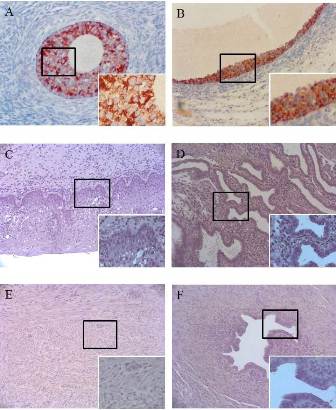
Fig. 1(A): In human ovary specimen, small antral follicle state, strongly express MIS in the granulose cell membrane. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X. (B) in human ovary specimen, antral follicle state, strongly express MIS in the granulose cell membrane. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X.(C) In human uterine cervix specimen, no reactivity for MIS Immunohistochemistry. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X. (D) In human uterine endometrium specimen, no reactivity for MIS Immunohistochemistry. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X. (E) In human uterine myometrium specimen, no reactivity for MIS Immunohistochemistry. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X. (F) In human uterine tube specimen, no reactivity for MIS Immunohistochemistry. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X.
MISRII expression via Immunohistochemistry
A strong MISRII expression was observed in the ovary at the follicular phase, which showed stronger expression as granulose cells of preantral follicles grew into multiple layers (Figure 2A). As preantral follicles were developed into antral follicles in the ovary at the follicular phase, the MISRII expression was observed not only in the granulose cells, but also in the theca cells. The MISRII expression was shown to be lower in the theca cells than in the granulose cells (Figure 2B). The MISRII expression was observed in a small number of cells in the Corpora Luteum and atretic follicles at the luteal phase. No MISRII expression was shown in the eggs, ovary stroma, atretic follicles, epithelium and corpus albicans for both follicular phase and luteal phase groups. In addition, a strong MISRII expression was observed in the uterine cervix (Figure 2C), uterine endometrium (Figure 2D), and fallopian tube (Figure 2F) for both follicular phase and luteal phase groups. The MISRII expression was shown to be lower in the uterine myometrium than in the other tissues (Figure 2E). In the uterine cervix, a strong MISRII expression was shown in the epithelium, whereas no expression was observed in the interstitial stromal cells (Figure 2C). In the uterine endometrium, a strong MISRII expression was shown in the epithelium, whereas no expression was observed in the basement membrane and interstitial stromal cells (Figure 2D). In the uterine myometrium, the MISRII expression was shown to be lower in the smooth muscle cells than in the other tissues (Figure 2E). In the fallopian tube, a strong MISRII expression was shown in the mucosal epithelium, whereas no expression was observed in the lamina propria and basement membrane (Figure 2F).
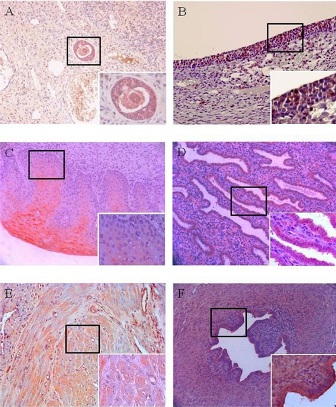
Figure 2(A): In human ovary specimen, small antral follicle state, strongly express MISRII in the cell membrane. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X. (B) In human ovary specimen, antral follicle state, strongly express MISRII in the cell membrane. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X.(C) In human uterine cervix specimen, strongly express MISRII in the cell membrane. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X. (D) In human uterine endometrium specimen, strongly express MISRII in the cell membrane. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X. (E) In human uterine myometrium specimen, mildly express MISRII in the cell membrane. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X.(F) In human uterine tube specimen, strongly express MISRII in the cell membrane. Right lower figure is higher magnification of the boxed area (400 X). Chromogen: AEC. Magnification, 200 X.
MISRII expression via RT-PCR
RT-PCR was conducted to examine the expression of MISRII mRNA in the ovary, uterine cervix, uterine endometrium, uterine myometrium, and fallopian tube. As a result, the expected 361 bp band was observed in all the tissues. The result of sequencing showed that the band was identical to some portion (581-941) of human MISRII cDNA sequence (Gene Bank, Accession No: AF172932). However, its expression level was shown to vary according to tissue. The expression was shown to be stronger in the ovary (A) than in the other tissues. The expression was moderately observed in the uterine cervix (B), uterine endometrium (C), and fallopian tube (E), whereas it was weakly observed in the uterine myometrium (D) (Figure 3).
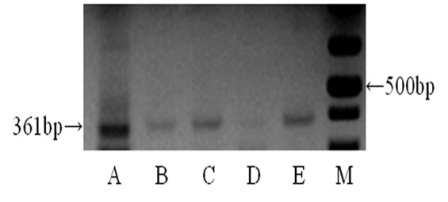
Figure 3: RT-PCT for human MISRII from human female genital tract. Bands of 361 bp are detected very strongly in ovary (A), and moderately detected in uterine cervix (B), uterine endometrium (C), and uterine tube (E). But from uterine myometrium (D) bands of 361 bp is mildly detected.
Discussion
MIS or AMH is produced in the immature Sertoli cells of the fetal testis, and then binds to the receptors present in the mesenchymal cells nearby mullerian duct, thereby degenerating mullerian duct. Via this mechanism, it inhibits the development of the fallopian tube, uterine, and upper vagina in men, which plays an important role in sex development. In addition, it is also involved in fatal lung maturation and testis differentiation and descending. In men, MIS is present at a concentration of 10-70 ng/m at the time of birth. Its concentration steadily increases until adolescence, and thereafter, decreases and maintains at a concentration of 2-5 ng/mL [5, 7]. In women, meanwhile, MIS is rarely produced before teenage. Since adolescence, it is produced in ovarian granulosa cells, and then secreted into the serum and follicular fluid at a concentration of 2-5 ng/mL, which is similar to that of men. It affects follicle selection, oocyte maturation, follicle development, and inhibition of steroid production in fertile women via autocrine, paracrine, and endocrine [6, 7]. As MIS is no more secreted after menopause, it can be used as a marker to measure the loss of ovarian functions [5, 7, 15].
MIS is a 140 kilodalton (kDa) glycoprotein consisting of 535 amino acids. It belongs to TGF-β multigene family such as TGF-β, inhibin, actibin, decapentaplegia, Vg1, bone morphogenetic protein (BMP), and growth and differentiation factor (GDF) [16]. rhMIS is a 70 kDa stripe-like glycoprotein due to the broken S-S bond. MIS gene is expressed at chromosome 19p in humans. It consists of 5 exons, of which 25 kDa C-terminus, which is identical among TGF-β family, is activated via proteolysis, thereby degenerating mullerian duct and inhibiting follicle growth [17] and 110 kDa N-terminus extends the half-life of MIS [18]. Human MIS structure shares 75% homology with that of cows. As it is also similar to that of rats, cows and rats are commonly used in studies [19].
For MIS role in the ovary, cyclic recruitment occurs by FSH that increases during the early follicular phase of menstruation, and approximately 10 antral follicles are selected and the remaining antral follicle become atretic follicles [23]. Androgen, which is produced in the theca cells, promotes ovarian follicular atresia by inhibiting FSH receptor expression in the granulosa cells and by reducing aromatase production. MIS, which is strongly expressed at that stage, has been known to inhibit aromatase production, and to block the conversion of androgen into estrogen, thereby increasing androgen in the follicles. Tetsuka et al., [24] also reported that the mRNA expression pattern of androgen receptor and MISRII mRNA were similar. Based on previous results showing that MISRII expression was first observed in the theca cells that mainly produces androgen at that stage, and that the selected follicles had a potent immune response, but no MIS expression was shown in the atretic follicles, MIS is likely to be involved in ovarian follicular atresia and follicle selection. Meanwhile, as the selected follicles have a high sensitivity to FSH, they continuously produce estrogen due to the increased aromatase activity. Thus, it can be interpreted that MIS and MISRII steadily decrease due to the increased FSH, and that they are further reduced due to the increased estrogen caused by the reduction of MIS inhibition activity [23]. At the follicular phase of menstrual cycle, as FSH decreases due to increases in estrogen and inhibin, a follicle, which has a highest concentration of FSH receptor survives among the selected follicles, becomes a dominant follicle, thereby growing into a mature follicle [25]. If it becomes a completely mature follicle, the restart of egg meiosis, and egg maturation and ovulation, which are inhibited at the early phase of primary meiosis, resume due to increased LH secretion.
MIS has been known to affect cell growth as well as sex development and menstruation inhibition. Accordingly, it has been speculated that MIS could inhibit tumor growth derived from mullerian duct. Thus, studies have been actively conducted to investigate the possible effect of MIS on the treatment or regulation of cancers in female patients with cancer of the reproductive system. In addition to female reproductive system, it has been suggested that MIS could act as a multifunctional cell regulator after the identification of MIS receptor [26, 27].
In this study, the expression of MIS and MISRII was examined in each tissue of female reproductive organs. The result showed that MIS was expressed only in the ovary among the female reproductive organs, where MISRII was expressed in all the female reproductive organs, but its expression level varied according to tissue. That is, the MISRII expression was high in the ovary, and intermediate in the uterine cervix, uterine endometrium, fallopian tube, and low in the uterine myometrium. As uterine myometrium, which is also derived from mullerian duct like the uterine cervix, uterine endometrium, and fallopian tube, had a different MISRII expression level, a further study on female menstrual mechanism is required. In addition, as this study was conducted using the tissues collected from fertile women aged 37-45 years; a further study is required to investigate changes in MISRII expression by subject’s age using the normal uterine tissue of pre-adolescent and post-adolescent women. However, considering that it is difficult to collect the normal uterine tissue from pre-adolescent and adolescent women, a study using animal model is recommended in advance. In this study, the normal uterine tissue was collected from the patients who underwent hysterectomy due to uterine myoma or uterine adenomyosis. Thus, an additional study on the MISRII expression of the lesions of uterine myoma and adenomyosis is required. If the MISRII expression pattern of uterine myoma or uterine adenomyosis differs from that of the normal uterine myometrium, it could be helpful for identifying the pathogenesis of these diseases. The results of this study could be used as a basis for understanding normal menstrual mechanism, MIS effect on menstrual mechanism, and the pathogenesis of uterine myoma and adenomyosis.
Conclusion
Finally we found that MIS is expressed only in ovary of female genital tract. And MISRII is presented on the entire female genital tract but the expression intensity was different relatively. These results suggest that MIS may have the function of biological modifier or inhibitor on female genital organ development and maturation. We could use this study for basic useful research to understand the physiology of MIS.
Authors' Contribution
HBL: Carried out the literature search and prepared the draft manuscript.
YJK: Carried out the literature search and prepared the draft manuscript
MJK: Helped in preparing the manuscript
HHC: Literature search manuscript preparation
MRK: preparing the draft manuscript
EJK: Manuscript preparation
JHK: Editing of the final manuscript
Conflict of Interests
The authors delcalre that there are no conflict of interests
Ethical Considerations
The study was approved by ethical committee
Funding
None
References
[1]. Jost A. Sur les derives mulleriens d'embyons de lapin dex deux sex’s castres a 21 jours. CR Biol 1947; 141:135-6. [Pubmed].
[2]. Picon R. Action of the fetal testis on the development in vitro of the Müllerian ducts in the rat. Arch Anat Microsc Morphol Exp 1969;58:1-19. [Pubmed].
[3]. Budzik GP, Powell SM, Kamagata S, Donahoe PK. Mullerian inhibiting substance fractionation by dye affinity chromatography. Cell 1983;34:307-14. [Pubmed].
[4]. Vigier B, Picard JY, Campargue J, Forest MG, Heyman Y, Josso N. Secretion of anti-Müllerian hormone by immature bovine Sertoli cells in primary culture, studied by a competition-type radioimmunoassay: lack of modulation by either FSH or testosterone. Mol Cell Endocrinol 1985; 43:141-50. [Pubmed].
[5]. Hudson PL, Dougas I, Donahoe PK, Cate RL, Epstein J, Pepinsky RB, MacLaughlin DT. An immunoassay to detect human müllerian inhibiting substance in males and females during normal development. J Clin Endocrinol Metab 1990; 70:16-22. [Pubmed].
[6]. Lee MM, Donahoe PK. Müllerian inhibiting substance: a gonadal hormone with multiple functions. Endocr Rev 1993; 14:152-64. [Pubmed].
[7]. Lee MM, Donahoe PK, Hasegawa T, Silverma B, Crist GB, Best S, Hasegawa Y, Noto RA, Schoenfeld D, MacLaughlin DT. Müllerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab 1996; 81:571-6. [Pubmed].
[8]. Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, Uilenbroek JT, Karels B, Wilming LG, Meijers JH, Themmen AP, Grootegoed JA. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Müllerian duct. Development 1994; 120:189-97. [Pubmed].
[9]. di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, Picard JY, Vigier B, Josso N, Cate R. Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol Endocrinol 1994; 8:1006-20. [Pubmed].
[10]. Richards JS. New signaling pathways for hormones and cyclic adenosine 3', 5’-monophosphate action in endocrine cells. Mol Endocrinol 2001; 15:209-18. [Pubmed].
[11]. Allard S, Adin P, Gouédard L, di Clemente N, Josso N, Orgebin-Crist MC, Picard JY, Xavier F. Molecular mechanisms of hormone-mediated Müllerian duct regression: involvement of beta-catenin. Development 2000; 127:3349-60. [Pubmed].
[12]. Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR. Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev 1996; 10:2577-87. [Pubmed].
[13]. Behringer RR, Finegold MJ, Cate RL. Müllerian-inhibiting substance functions during mammalian sexual development. Cell 1994; 79:415-25. [Pubmed].
[14]. Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 1999; 140:5789-96. [Pubmed].
[15]. Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod 2005; 20:923-7. [Pubmed].
[16]. Akhurst RJ, FitzPatrick DR, Gatherer D, Lehnert SA, Millan FA. Transforming growth factor betas in mammalian embryogenesis. Prog Growth Factor Res 1990; 2:153-68. [Pubmed].
[17]. MacLaughlin DT, Hudson PL, Graciano AL, Kenneally MK, Ragin RC, Manganaro TF, Donahoe PK. Müllerian duct regression and antiproliferative bioactivities of Müllerian inhibiting substance reside in its carboxy-terminal domain. Endocrinology 1992; 131:291-6. [Pubmed].
[18]. Catlin EA, MacLaughlin DT, Donahoe PK. Müllerian inhibiting substance: new perspectives and future directions. Microsc Res Tech 1993; 25:121-33. [Pubmed].
[19]. Cohen-Haguenauer O, Picard JY, Mattéi MG, Serero S, Nguyen VC, de Tand MF, Guerrier D, Hors-Cayla MC, Josso N, Frézal J. Mapping of the gene for anti-Müllerian hormone to the short arm of human chromosome 19. Cytogenet Cell Genet 1987;44:2-6. [Pubmed].
[20]. Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, Jung M, MacLaughlin DT, Donahoe PK, Maheswaran S. Müllerian inhibiting substance inhibits breast cancer cell growth through an NFκB-mediated pathway. J Biol Chem 2000; 275:28371-9. [Pubmed].
[21]. Kretzschmar M, Massagué J. SMADs: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev 1998; 8:103-11. [Pubmed].
[22]. Hirobe S, He WW, Lee MM, Donahoe PK. Müllerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology 1992; 131:854-62. [Pubmed].
[23]. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21: 200-14. [Pubmed].
[24]. Tetsuka M, Whitelaw PF, Bremner WJ, Millar MR, Smyth DD, Hillier SG. Developmental regulation of androgen receptor in rat ovary. J Endocrinol 1995; 145: 533-43. [Pubmed].
[25]. Kuroda T, Lee MM, Ragin RC, Hirobe S, Donahoe PK. Müllerian inhibiting substance production and cleavage is modulated by goandotropins and steriods. Endocrinology 1991; 129: 2985-92. [Pubmed].
[26]. MacLaughlin DT, Donahoe PK. Müllerian inhibiting substance/anti-Müllerian hormone: a potential therapeutic agent for human ovarian and other cancers. Future Oncol 2010; 6:391-405. [Pubmed].
[27]. Hwang SJ, Suh MJ, Yoon JH, Kim MR, Nam SW, Donahoe PK, Maclaughlin DT, Kim JH. Identification of characteristic molecular signature of Müllerian inhibiting substance in human HPV-related cervical cancer cells. Int J Oncol. 2011 Oct;39(4):811-20. [Pubmed].
[28]. Kim JH, Jae DS, Kim TE, Shin JI, Kim EJ, Lee JW, Na JG, Kim SP. The effect of Müllerian Inhibiting Substance on Steroidogenesis and Proliferation of Human Granulosa Cells. Korean Journal of Obstetrics and Gynecology. 1996; 39: 2294-303.
[29]. Kim JH, Chung SH, Choi EJ, Hwang SJ, Jo HH, Kim MR, Kim EJ, Kim JH, Ryu KS. Expression of Müllerian Inhibiting Substance and Its receptor in the Human ovary during menstrual cycle. Korean Journal of Obstetrics and Gynecology. 2004 ; 47 : 1725-1732
[30]. We JS, Song JY, Kim SY, Jo YS, Jo HH, Kim MR, Kim JH, Jim JH. Müllerian Inhibiting Substance as a predictive marker of menopausal transition. Korean Journal of Obstetrics and Gynecology. 2007 ; 50 : 1396-1404
[31]. Gleicher N, Weghofer A, Barad DH. Discordances between follicle stimulating hormone (FSH) and anti-Müllerian hormone (AMH) in female infertility. Reproductive Biology and Endocrinology 2010; 17;8: 64. [Pubmed].
[32]. La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS. Anti-Müllerian hormone (AMH): what do us stillneed to know? Human Reproduction 2009; 24, 2264-75. [Pubmed].
[33]. Imbeaud S, Faure E, Lamarre I, Mattei MG, di Clemente N, Tizard R, Carre-Eusebe D, Belville C, Tragethon L, Tonkin C. Insensitivity to anti-Müllerian hormone due to a mutation in the human anti-Müllerian hormone receptor. Nature Gen 1995; 11: 382-8. [Pubmed].
[34]. Josso N. Pediatric applications of anti-Müllerian hormone research. Horm Res 1995; 43: 243-248. [Pubmed].
[35]. Kim JH, Seibel MM, MacLaughlin DT, Donahoe PK, Rabsil BJ, Hametz PA, Richards CJ. The inhibitory effects of Müllerian inhibiting substance on epidermal growth factor induced proliferation and progesterone production of human granulosa-luteal cells. J Clin Endocrinol Metab 1992; 75: 911-7. [Pubmed].

