Case Report

Xanthogranuloma of the sellar region in a patient with acromegaly. Case report
1Vita Stagno, 2Sara Baldo, 3Marialaura Del Basso De Caro, 1Felice Esposito, 1Luigi M. Cavallo, 1Paolo Cappabianca
- 1Department of Neurological Sciences, Division of Neurosurgery, Università degli Studi di Napoli Federico II Naples, Italy
- 2Division of Neurosurgery, A.O.U. “Ospedali Riuniti”, Trieste, Italy
- 3Department of Biomorphological and Functional Sciences, Section of Pathology, Università degli Studi di Napoli Federico II Naples, Italy
- Submitted: November 19, 2012, 2012
- Accepted: January 16, 2013
- Published:March 01, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Xanthogranuloma is a rare pathological entity, recently added to the WHO brain tumor classification.
Case report
A 22-year-old man was admitted to our Department with a history of progressive growth of acral extremities and mandibular prognatism. Clinical symptoms and blood-tests were suggestive of acromegaly. A sellar MRI revealed an intrasellar mass extending into the suprasellar region, consistent with the diagnosis of pituitary macroadenoma. A preoperative treatment with somatostatin-analogs for a year caused the suppression of the augmented growth hormone, but no reduction of the tumor size. The patient was then operated by means of an endoscopic endonasal transphenoidal approach. Histological examination revealed the presence of granulomatous tissue with cholesterol clefts, marked hemosiderin deposits and no epithelial component. The final diagnosis was of xanthogranuloma of the sellar region. A three and eighteen month post-op MRI confirmed the total tumor removal, while endocrinological test revealed the normalization of GH blood values.
Conclusion
This is the first case reported in the literature of a xanthogranuloma of the sellar region occurred in a patient with acromegaly.
Key words
xanthogranuloma sellar region; acromegaly; cholesterol granuloma; endoscopic pituitary surgery, sellar region tumors; transsphenoidal surgery.
Introduction
The xanthogranuloma is a lesion described in different cranio-facial locations (middle ear, mastoid bone, paranasal sinuses), sometimes as a part of a systemic disease [4, 6, 11, 13, 17, 18, 20], occasionally even in dogs [9]. Among the different intracranial localizations reported, the sellar region seems to be the most common, but few cases are reported in the literature [1, 10, 14, 17, 22, 23].
A first description of the xanthogranuloma of the sellar region was done by Paulus in 1999 [16]. In 2000, the lesion was finally inlcuded as a specific entity by the WHO (World Health Organization). Its prevalence appears to peak at 20-40 years of age. Typically the xanthogranuloma causes symptoms of pituitary dysfunction or visual change due to compression of the optic chiasm. The histological features of the lesion are characterized by the presence of cholesterol-clefts granulomas and hemosiderin deposits [17].
Actually, no case of xanthogranuloma-associated with acromegaly has been documented in the literature. We report the clinical and histopathological features of a xanthogranuloma of the sellar region in a young patient with history of acromegaly.
Case report
A 22-year-old man presented with a history of progressive growth of acral extremities as well as mandibular prognatism. He also had been complaining headache during the last two months. The neurological examination was unremarkable, while the physical examination showed the typical acromegalic features. There were no clinical signs of compression of the optic chiasm or pituitary deficiency. The blood-test confirmed the elevated basal GH (8.7 ng/ml [normal range 0.1-5 ng/ml]) and IGF-1 levels [1226 ng/ml [normal range 80-400 ng/ml]). A contrast-enhanced brain MRI revealed a sellar mass, which determined an enlargement of the sella turcica, with upward growth and dislocation of the pituitary stalk and impingement of the optic chiasm. At the basal images, the aspect of the tumor was homogeneous, isointense with the brain and, after contrast administration, the lesion showed a nonhomogeneous and late enhancement (Fig. 1 A-B). Therefore, the patient underwent a 9-month therapy with somatostatin-analogs (longastatine LAR 120 mg every 3 weeks) with subsequent normalization of IGF-1 (275 ng/mL) and GH (0, 9 ng/mL). Despite the medical treatment, the MRI at 1 year revealed the persistence of the lesion. (Fig.1C-D). The hormone levels at 13 months revealed a new increase of the IGF-1 (755 ng/mL) while the GH remained within the normal range (2.26 ng/mL). The patient was then scheduled for surgery with the preoperative diagnosis of GH-secreting pituitary macroadenoma. He underwent a selective lesion removal by means of an endoscopic endonasal transsphenoidal approach. Macroscopically, the tumour appeared to be yellow-brownish, soft, with a fluid component, capsulated and well isolated from the residual gland, which was identified posteriorly in the left side of the sellar cavity (Fig. 2A-B).
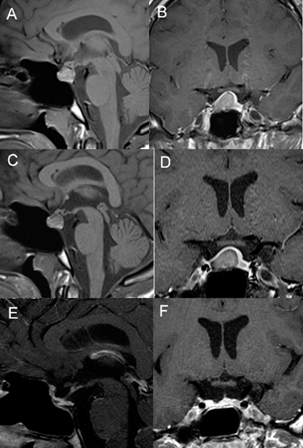
s
Fig 1 A-B: T1-weighted sagittal and coronal sellar MRI revealed a right-side sellar lesion, compressing the pituitary gland and displacing the pituitary stalk; C-D: 1 year post-medical therapy T1-weighted sagittal and coronal images MRI Revealed the persistence of the lesion without any relevant modifications; E-F: Three month T1-weighted post-operative MRI showing the removal of the xantogranuloma with preservation of the pituitary gland.
Neuropathological examination revealed fibrovascular tissue with hemorrhagic extravasation, cellular infiltrates of plasmacells, granulocytes and lymphocytes, numerous cholesterol clefts (Fig. 3A) and multinucleated giant cells (Fig. 3B). Focally, there were also acini and nests of adenohypophysial cells, some acidophilic or basophilic, others cromophobic (Fig. 3C). Further immunohistochemical analyses showed that such cells expressed in different proportions all the tested adenohypophysial hormones; moreover, this pituitary tissue retained normal acinar architecture of reticulin fiber network (Fig. 3D). No features corresponding to pituitary adenoma have been found in the histological sections.
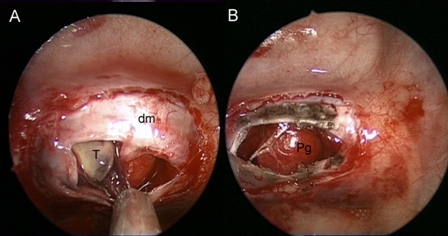
Fig. 2A: Intraoperative images showing a grey-brownish soft lesion located in the right portion of the sella and the normal pituitary gland, on the left side; B: after the tumor removal, the residual pituitary gland appears preserved and decompressed. Dm: dura mater; Pg: pituitary gland; T: tumor.
The postoperative course was uneventful, and the patient was discharged on postoperative day 3. A new endocrinological test revealed normal GH blood values (0, 65 ng/mL) and IGF-1 (260 ng/mL), while a three month post-op MRI confirmed the total tumour removal with preservation of the pituitary gland (Fig 1E-F). A new clinical and neuroradiological follow-up, 18 months after surgery, did not show any tumour recurrence and the patient is currently well without any medication.
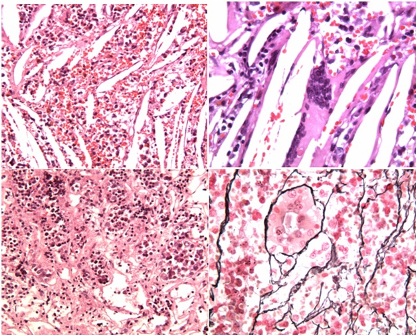
Fig. 3A: Cholesterol clefts, hemorrhagic extravasation and dense inflammatory infiltrate (H/E 20x); B: note multinucleated giant cell (H/E 40x); C: perilesional localization of acini and nests of adenohypophysial cells. Some cells are acidophilic or basophilic, others are cromophobic (H/E 20x); D: reticulin fiber network retains normal acinar architecture (reticulin staining 40x).
Discussion
A cholesterol granuloma reaction in intrasellar lesion was first described in 1988 as a possible evolution of adenoma (secondary to necrosis or hemorrhage) or craniopharyngioma (process of keratinization) [17]. In 1999, Paulus et al., in a series of 110 cases of craniopharingioma, reported 37 patients with distinct histological features that appeared to have a predominating xanthogranulomatous component without epithelial cells [16]. This description was then added to the WHO brain tumor classification as distinct entity and named “xanthogranuloma of the sellar region” [12].
It occurs predominantly in young adults, in their first decades of life, but some cases have also been reported in the fifth and sixth decade. This rare lesion typically causes symptoms of pituitary dysfunction or visual changes (due to compression of the optic chiasm). There is a unique report that documented a case of xanthogranuloma presented with symptoms of hydrocephalus [14]. There are no typical radiological signs known for xanthogranuloma. However, cholesterol and therefore cholesterol clefts appear hyperintense in T1-weighted images but focal hemorrhage and hemosiderin deposits can result in inhomogeneous contrast enhancement. The majority of reported xanthogranuloma cases were initially misjudged as craniopharyngiomas or pituitary adenomas [16]. Further differential diagnoses of xanthogranuloma of the sella are: Rathke’s cleft cysts, metastases, meningiomas, hypophysitis as well as intrasellar aneurysms. Liu reported that a pre-operative diagnosis of xanthogranuloma is very difficult [14].
However, to the best of our knowledge, no case of xanthogranuloma-associated with acromegaly has been documented in the literature (Table 1).
Table 1: Metanalysis of the literature on xanthogranuloma cases.
|
Author |
Cases |
Location |
MRI |
Clinical
symptoms |
Surgical
approach |
Follow-up |
Yonezawa
(2003) |
1 |
Intrasellar |
Hyperintense;
no
enhancement |
panhypopituitarism |
Transsphenoidal |
hormonal
replacement |
Burt
(2003) |
2 |
Intrasellar |
Heterogeneous
enhancement; |
hypopituitarism |
Transsphenoidal/
Transcranial |
hormonal
replacement |
Jung
(2006) |
2 |
Intrasellar |
Heterogeneous
enhancement; |
hypopituitarism |
Transsphenoidal/
Transcranial |
visual field
improvement |
Liu
(2007) |
1 |
Sellar-suprasellar |
Hyperintense
cystic mass |
drowsiness |
Transcranial |
Improvement of
neurological function |
Sugata
(2009) |
1 |
Suprasellar |
Heterogeneous
enhancement |
bitemporal
hemianopia |
Transcranial |
visual field
improvement |
Sulentic
(2010) |
1 |
Sellar-suprasellar |
- |
panhypopituitarism |
Transsphenoidal |
- |
Arai
(2010) |
1 |
Sellar-suprasellar |
Homogeneously
hyperintense |
hypopituitarism |
Transsphenoidal |
hormonal
replacement |
Present
report |
1 |
Sellar-suprasellar |
Heterogeneous
enhancement |
Acromegalic
features |
Transsphenoidal |
cured |
The primary objective of therapy in acromegaly is to reverse symptoms and signs of the disease; somatostatin-analogs have been considered a cornerstone of the medical therapy; they induce a further decrease of GH and IGF-1 levels and tumor shrinkage [5, 7, 8, 15]. Long-term therapy with somatostatin-analogs favored, in certain patients, a granulomatous reaction on the tumor cells [2, 7]. Usually the presence of haemosiderine deposits, at the histopathologic exam, suggests an hemorrhage into the primary lesion; nevertheless, in this case the histological examination revealed no signs compatible with the diagnosis of GH-secreting adenoma and on the contrary, peculiar characteristics for the diagnosis of xanthogranuloma. We don’t believe that changes induced by the pharmacological therapy may have played a pathogenic role in the development of xanthogranuloma. So a possible explanation would be the co-existence of two different lesions (xantogranuloma and pituitary microadenoma). Hence it could had happen that during the tumor removal an already small and hidden GH secreting adenoma, was accidentally suctioned leaving only indirect signs of its presence.
Conclusion
Based on what is reported in the literature and on our clinical findings, we believe that the characteristic aspect, which we histologically noted, cannot represent an evolution depending from the pharmacological therapy. Conversely, it may correspond to the remnant of a GH-secreting adenoma coexisting with a xantogranulomatous lesion. Neverthless, the rarity of xanthogranulomatous lesions have limited the understanding of their natural history and pathogenesis and we have reported it for the coexistence of the acromegalic syndrome never seen before in previous reports.
Authors' Contribution
LMC, DBDC: Conception and design.
SB, VS: Acquisition of data, Analysis and interpretation of data, drafting the article.
FE, LMC: Critically revising the article.
PC: Reviewed final version of the manuscript and approved it for submission.
LMC: Study supervision.
Conflict of Interests
The Authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical Considerations
This work has been approved by the Ethical Committee of Department of Neurosurgery of the A.O.U. “Federico II” of Naples.
Funding
The Authors have nopersonal financial or institutional interest in any of the drugs, materials, or devicesdescribed in this article.
Acknowledgement
None
References
[1]. Arai A, Nishihara M, Sasayama T, Aihara H, Hosoda K, Itoh T, Sakagami Y, Kuwamura K, Kohmura E. Xanthogranuloma of the sellar region--case report. Neurol Med Chir (Tokyo) 2010; 50:488-91. [Pubmed].
[2]. Auriemma RS, Galdiero M, Grasso LF, Vitale P, Cozzolino A, Lombardi G, Colao A, Pivonello R. Complete disappearance of a GH-secreting pituitary macroadenoma in a patient with acromegaly: effect of treatment with lanreotide Autogel and consequence of treatment withdrawal. Eur J Endocrinol. 2010; 162:993-9. [Pubmed].
[3]. Burt MG, Morey AL, Turner JJ, Pell M, Sheehy JP, Ho KK. Xanthomatous pituitary lesions: A report of two cases and review of the literature. Pituitary 2003; 6:161-8. [Pubmed].
[4]. Cao D, Ma J, Yang X, Xiao J. Solitary juvenile xanthogranuloma in the upper cervical spine: case report and review of the literatures. Eur Spine J. 2008; 17:318-23. [Pubmed].
[5]. Cappabianca P, Cavallo LM, Esposito F, Romano I, Colao A, de Divitiis E. Rationale of pre-surgical medical treatment with somatostatin analogs in acromegaly. J Endocrinol Invest. 2003; 26:S55-8. [Pubmed].
[6]. Cauro F, Houtteville JP, Mesnil JL, Guarnieri J. Cerebellar, pulmonary and cutaneous, localizations of juvenile xanthogranuloma. Ann Dermatol Venereol. 2002; 129:307-10. [Pubmed].
[7]. Colao A, Auriemma RS, Rebora A, Galdiero M, Resmini E, Minuto F, Lombardi G, Pivonello R, Ferone D. Significant tumour shrinkage after 12 months of lanreotide Autogel-120 mg treatment given first-line in acromegaly. Clin Endocrinol (Oxf). 2009; 71:237-45. [Pubmed].
[8]. Colao A, Pivonello R, Auriemma RS, Galdiero M, Savastano S, Lombardi G. Beneficial effect of dose escalation of octreotide-LAR as first-line therapy in patients with acromegaly. Eur J Endocrinol. 2007; 157:579-87. [Pubmed].
[9]. Cramer SD, Miller AD, Medici EL, Brunker JD, Ritchey JW. Sellar xanthogranuloma in a dog. J Vet Diagn Invest. 2011; 23:387-90. [Pubmed].
[10]. Jung CS, Shänzer A, Hattingen E, Plate KH, Seifert V. Xantogranuloma of the sellar region. Acta Neurochir (Wien). 2006; 148: 473-7. [Pubmed].
[11]. Kim EJ, Kim MY, Kim HO, Park YM. Juvenile xanthogranuloma of the finger: an unusual localization. J Dermatol. 2007; 34:590-2. [Pubmed].
[12]. Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002; 61(3):215-29. [Pubmed].
[13]. Kolivras A, Theunis A, de Saint-Aubain N, Zelger B, Sass U, Dangoisse C, André J. Congenital disseminated juvenile xanthogranuloma with unusual skin presentation and renal involvement. J Cutan Pathol. 2009; 36:684-8. [Pubmed].
[14]. Liu ZH, Tzaan WC, Wu YY, Chen HC. Sellar xantogranuloma manifesting as obstructive hydrocephalus. J Clin Neurosci. 2008; 15: 929-33. [Pubmed].
[15]. Losa M, Ciccarelli E, Mortini P, Barzaghi R, Gaia D, Faccani G, et al,. Effects of octreotide treatment on the proliferation and apoptotic index of GH-secreting pituitary adenomas. J Clin Endocrinol Metab. 2001; 86:5194–5200. [Pubmed].
[16]. Paulus W, Honegger J, Keyvani K, Fahlbusch R. Xanthogranuloma of the sellar region: a clinicopathological entity different from adamantinomatous craniopharyngioma. Acta Neuropathol. 1999; 97:377-82. [Pubmed].
[17]. Shirataki K, Okada S, Matsumoto S. Histopathological study of the “cholesterol granuloma reaction” in the sellar and juxta-sellar tumors. No to Shinkei. 1988; 40: 133-9. [Pubmed].
[18]. Sugata S, Hirano H, Yatsushino K, Yunoue S, Nakamura K, Arita K. Xantogranuloma in the suprasellar region. Case report. Neurol Med Chir (Tokyo). 2009; 49:124-7. [Pubmed].
[19]. Sulentić P, Cupić H, Cerina V, Vrkljan M. Xanthogranuloma of the sellar region in a patient with sarcoidosis. Acta Clin Croat. 2010; 49:61-5. [Pubmed].
[20]. Thevasagayam MS, Ghosh S, O'Neill D, Panarese A, Bull PD. Isolated juvenile xanthogranuloma of the subglottis: case report. Head Neck. 2001; 23:426-9. [Pubmed].
[21]. Yokoyama S, Sano T, Tajitsu K, Kusumoto K. Xanthogranulomatous hypophysitis mimicking a pituitary neoplasm. Endocr Pathol. 2004; 15:351-7. [Pubmed].
[22]. Yonezawa K, Shirataki K, Sakagami Y, Kohmura E. Panhypopituitarism induced by cholesterol granuloma in the sellar region. Case report. Neurol Med Chir (Tokyo). 2003; 43: 259-62. [Pubmed].
[23]. Zada G, Lin N, Ojerholm E, Ramkissoon S, Laws ER. Craniopharyngioma and other cystic epithelial lesions of the sellar region: a review of clinical, imaging, and histopathological relationships. Neurosurg Focus. 2010; 28:4. [Pubmed].

