Case Report

Primary small cell carcinoma of the renal pelvis: a case report with review of literature
1Priti P. Trivedi, 1Divya Kriplani, 1Ashini Shah, 2Sanjoy Sen, 2Hemang Baxi, 1M. J. shah
- 1Department of pathology, The Gujarat cancer and research institute, Ahmedabad
- 2Department of surgical oncology, The Gujarat cancer and research institute, Ahmedabad
- Submitted: March 21, 2013;
- Accepted March 28, 2013;
- Published: April 08, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Primary small cell carcinomas originating in the renal pelvis are extremely rare and very aggressive neoplasms.
Case report
We report the case of a 70 year old male who presented with hematuria and flank pain. The patient underwent a left nephroureterectomy with left Para aortic lymph node dissection. Histopathology and immunohistochemistry revealed a small cell carcinoma of the renal pelvis. The patient refused chemotherapy and died two months following surgery.
Conclusion
Small cell carcinoma of the renal pelvis is a rare and aggressive neoplasm with limited survival due to a high incidence of occult metastasis at the time of presentation. After a comprehensive literature search we found 14 cases of small cell carcinoma of the renal pelvis including the current case, and a review of the same is presented and discussed.
Key words
small cell carcinoma, kidney, renal pelvis, prognosis.
Introduction
Extra pulmonary small cell carcinoma (EPSCC) is rare and comprises only 2.5% of all cases of small cell carcinoma [1]. EPSCC has been reported in nearly all organs except the central nervous system and the liver, with the most common site being the urinary bladder [1, 2]. Primary renal small cell carcinoma (SCC) is rare and to date approximately 50 cases have been reported in the world literature [3]. Renal SCC may occur in either the renal pelvis or the renal parenchyma. SCC occurring in the renal pelvis has distinct features in that they occur in association with neuroendocrine component like transitional cell carcinoma (TCC), adenocarcinoma, squamous cell carcinoma and rarely carcinoid [4]. We present a case of SCC of the renal pelvis in a 70 year old male.
Case report
A 70 year old male presented with the chief complaints of gross hematuria, increased frequency of micturition and left flank pain since two years. The patient was a chronic smoker. A computed tomography (CT) scan and intravenouspyelography (IVP) were done. The left kidney was 12X5.5 cms in size with delayed contrast excretion. There was moderate hydronephrosis, and an enhancing mass lesion measuring 4.7X2.6X2.5cms was seen in the left renal pelvis and upper ureter. The right kidney measured 11.7X4.7 cms and showed normal contrast excretion. Right pelvicalyces were normal. Right ureter was normal in course and caliber. Multiple enlarged nodes were seen in the left paraaortic region, largest measuring 3.2X1.7 cms in size. Urinary bladder, prostate, seminal vesicles and adrenal glands were normal. Liver, spleen and pancreas were also normal. Ascites was absent.
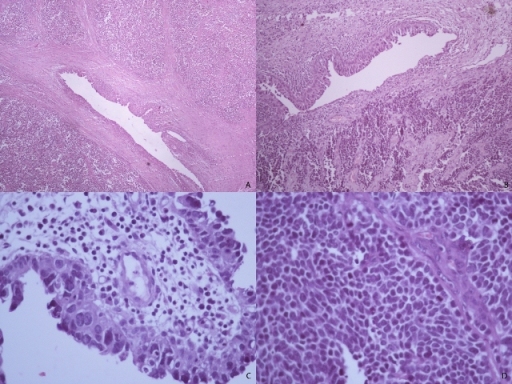
Figure 1: Small cell carcinoma of the renal pelvis. (A&B) low power view shows renal pelvis epithelium with proliferation of small round cells (H&E, 40X). (C) Transitional cell carcinoma in situ of the renal pelvis (H&E, 40X). (D) High power view of the small cell carcinoma area (H&E, 40X).
A DTPA (Diethylene Triamine Pentathenic Acid) renal scan was done and showed a hydronephrotic left kidney with moderately reduced relative function (24.39%). There was steady accumulation of the tracer seen at the pelviureteric junction (PUJ) suggestive of PUJ obstruction. Right kidney was well visualized with good cortical function and unobstructed drainage. A CT angiography for inferior vena cava did not show any tumor thrombus. The metastatic work up was negative. With the above findings in hand, the patient underwent a left nephroureterectomy with left paraaortic lymph node dissection.
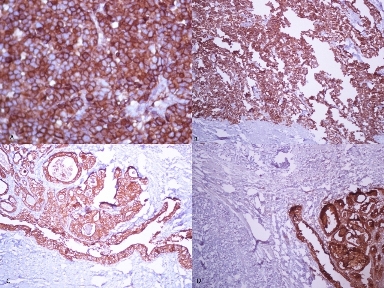
Figure 2: Immunohistochemistry of small cell carcinoma of the renal pelvis. (A) Strong synaptophysin positivity (40X). (B) Strong chromogranin positivity (40X). (C) AE1 positivity in the transitional cell carcinoma component (10X). (D) Epithelial membrane antigen (EMA) positivity in transitional cell carcinoma component (10X).
Pathology findings
Grossly, a 4.5X3.5X2.5 cms sized polypoidal growth was found involving the left renal pelvis and upper ureter with gray white to brown cut surface. Necrosis was evident. Tumor was close to the hilar fat. Hydronephrosis was seen. Rest of the left renal parenchyma was grossly unremarkable. Cut end ureter was 9.5 cm away and free from the tumor. Cut ends of renal vessels were also free of tumor. Several lymph nodes were found, largest measuring 3.5 cm in size with metastatic cut surface grossly.
Histopathology revealed a small cell carcinoma with monomorphic tumor cells having scanty cytoplasm and dispersed chromatin. Nuclei were hyperchromatic with nuclear molding and inconspicuous nucleoli. Brisk mitotic activity was seen. Extensive lymph vascular permeation was evident. The tumor infiltrated into the wall of the ureter and involved the hilar fat. There was evidence of transitional cell carcinoma in situ of the renal pelvis. Immunohistochemistry showed cytokeratin, synaptophysin and chromogranin positivity while CK7, CK20, TTF1 and vimentin were negative. Total five lymph nodes were found, one out of these five lymph nodes showed metastatic small cell carcinoma. The patient was advised adjuvant chemotherapy but refused. Patient died two months later.
Discussion
Extrapulmonary small cell carcinomas are rare, while primary small cell carcinomas arising from the kidney are even more unusual.
The diagnosis of renal SCC is based on the light microscopic criteria established for the diagnosis of pulmonary SCC. Light microscopy reveals small and oval, round to spindle shaped cells having up to twice the diameter of normal lymphocytes, hyper chromatic nuclei, inconspicuous nucleoli, nuclear molding, scant cytoplasm and increased mitotic activity (>11/10 HPF). On electron microscopy, the presence of membrane bound neurosecretory granules and desmosomes is most consistent with SCC. Neuron specific enolase (NSE), chromogranin and synaptophysin are the most commonly used neuroendocrine markers in the evaluation of renal SCC.
Total fourteen cases including the current case of SCC of the renal pelvis, excluding cases of small cell carcinoma of the renal parenchyma, have been reported in the world literature to date (Table 1) [5-15]. Two case reports from china with English language abstract are included (case 8 and 9) while one case report from France without abstract is excluded from this review [16].
Table 1: Summary of published cases of small cell carcinoma of the renal pelvis.
| Patient no. |
Author, Year |
Age(y) sex |
History and Investigations |
Histopathology and Ultrastructural findings |
Treatment |
Follow up |
| 1) |
Mills et al., 1988 (5) |
66, M |
Left flank pain and hematuria |
4.7 cm tumor, SCC with TCC in situ |
Left Nephrectomy |
DOD, 6 months |
| 2) |
Essenfeld et al., 1990 (6) |
66, F |
Intermittent hematuria, tiredness. Phenacitin abuse 10- Years .IVP Left kidney not visualized. RGP Left renal mass |
7x3x2 cm papillary mass in the left renal pelvis, no infiltration into the renal parenchyma, SCC with grade III TCC |
Left Nephrectomy |
DF, 16 months TCC in the opposite renal pelvis |
| 3) |
Essenfeld et al.,1990 (6) |
62, F |
Anorexia, asthenia, right flank discomfort and recurrent cystitis x3 weeks. Heavy smoker, B/I nephrolithiasis x 34 years. IVP right renal mass, multiple staghorn calculi. CECT scan- right renal mass, no metastasis. |
SCC with grade III papillary TCC |
Right nephrectomy -adjuvant CTx- Vinblastine and mitimycin - poor response -cyclophosphamide and 5- Flurouracil |
DOD; lung metastases, 8 months |
| 4) |
Guillou et al., 1993 (7) |
71, F |
Intermittent Ruq pain x 2 months .Smoker, 50 pack-years .US and CECT scan right renal pelvis tumor, no metastases. Needle Biopsy- SCC |
5 cm tumor in the renal pelvis, SCC with TCC. Scarce neurosecretory granules, Plentiful desmosomes |
Right nephrectomy adjuvant CTx;6 cycles of carboplatin + teniposide -regional LN recurrence 3 months after surgery -RT 50 Gy to renal bed and scalp metastases |
DOD; 8 months |
| 5) |
Mazzucchelli et al., 1995 (8) |
37 F |
Gross hematuria x weeks. Smoker 1 PPD. CECT scan 15 x10x8 cm right renal pelvis tumor infiltrating into the perirenal fatty tissue, retroperitoneal LN metastases |
Undiffentiated SCC with rare foci of grade III TCC, tumor infiltrating fatty tissue, retroperitoneal LN metastases seen. neurosecretory granules seen |
Right nephrectomy Adjuvant Ctx; cyclophosphamide |
DOD; local progression and liver metastases, 3 months |
| 6) |
Kuromatsu et 78, al., 1995 (9) |
78, M |
Gross hematuria. CECT scan and RGP- right renal pelvis tumor |
SCC with grade II TCC, scattered desmosomes seen |
Right radical nephroureterectomy |
DOD; peritoneal carcinomatosa liver and LN metastases, 7 months |
| 7) |
Kitamura et 83, al., 1997 (10) |
83 F |
Right back pain with hematuria x 2 months; USG, IVP, CT scan - right kidney lower pole tumor, Urine Cytology class V TCC |
SCC with squamous and glandular differentiation, venous and lymphatic invasion seen |
Right nephrectomy |
DOD; systemic metastases, 2 months |
| 8) |
Kojima et al., 1998 (11) |
61, F |
Left lumbar pain, gross hematuria and high fever,CECT scan - Left renal pelvis tumor infiltrating the kidney with hilar lymphadenopathy |
SCC ;neurosecretory granules seen |
Neoadjuvant CTx; methotrexate, vinblastine, doxorubicin and cisplatin (M-VAC) Left nephrectomy |
DOD, 3 months |
| 9) |
Chuang and Liao 2003 (12) |
42, M |
Hematuria |
SCC with TCC |
Nephroureterectomy |
DOD; lung mets , 6 months |
| 10) |
Chuang and Liao 2003 (12) |
44, F |
Hematuria and pain |
SCC with TCC |
Nephroureterectomy |
DOD; lung bone and LN metastases 31 months |
| 11) |
Shimasaki et al., 2005 (13) |
61, F |
Right flank pain, microhematuria, progressive renal dysfunction. Urine Cytology TCC. US and CT scan right kidney- middle pole tumor . RGP right renal pelvis- tumor extending into PUJ |
6.5x4x3 cm tumor in the right renal pelvis SCC with sarcomatoid squamous cell carcinoma ,no extra renal invasion seen |
Righr radical nephroureterectomy with lymph node dissection |
DF, 11 months |
| 12) |
Banerji et al., 2008 (14) |
55. M |
Right flank pain x 6 months, CECT- 1.5 x1.5 cm pelvi- calyceal lesion and ureteric thickening 12 cm from the renal hilum with para- aortic and interaortacaval lymphadenopathy |
1x1x2 cm tumor in the renal pelvis, demonstrated only small cell carcinoma component and 7x1x1.5 cm tumor in the ureter had both transitional cell and small cell components No lymph node metastases |
Radical right nephroureterectomy with lymph node dissection Adjuvant CTx; gemcitabin and carboplatin |
Not Mentioned |
| 13) |
Sachin patil et al., 2011 (15) |
75, M |
Low back pain x 2 weeks. MR imaging- 4.8x4x3.7 cm homogeneous mass in the left renal pelvis with mild contrast enhancement and preaortic, para aortic,aortocaval and retrocaval lymphadenopathy |
Multifocal SCC with tumor emboli in the renal artery and lymphatics, tumor extended beyond fascia Gerota 4/4 LN positive for metastases |
Radical right nephroureterectomy with lymph node dissection |
Died of pneumonia 2 monhs after surgery |
| 14) |
Current Patient |
70/M |
hematuria,low back pain,incresed frequency of micturition(2 yrs). CT scan- 4.7x2.6x2.5 cm mass in left renal pelvis and upper ureter with paraaortic adenopathy, no distal metastases. |
4.5x3.5x2.5cm3 polypoidal growth of the left renal pelvis showing SCC with TCC in situ, infiltrating wall of the ureter and involving perirenal fat |
Radical left nephroureterectomy with lymph node dissection . Adjuvant CTx; |
DOD; 2 months |
The patients age ranged from 37 to 83 years with M:F ratio of 1:1.3. Past history of smoking was present in four (28.6%) cases. Gross hematuria was the most common symptom (71.4%) followed by pain (64.3%). No patient had an abnormal mass at presentation. Urine cytology was done in three cases. Two were reported as transitional cell carcinoma. Preoperative biopsy was done in only one patient (7.1%) and it was reported as SCC.
Three cases (21.4%), including the present one had locally advanced disease and lymph node metastases. No patient had systemic metastases or paraneoplastic syndrome at the time of presentation.
Histopathology results including local spread and lymph node metastases were available for 11 cases. A non neuroendocrine component was present in 12 of 14 patients. In one case (case 8), the histopathological findings were not clearly reported. TCC was the most common non neuroendocrine component. One patient had squamous and glandular components and another had squamous cell carcinoma component with sarcomatoid differentiation.
Surgery was performed in all cases. Seven patients (50%) underwent a nephrectomy and the other seven (50%) had a nephroureterectomy. Adjuvant chemotherapy was given to four patients and neoadjuvant chemotherapy was given to one patient. Palliative radiotherapy was used in one patient for local recurrence and scalp metastases. Six patients (42.8%) developed metastases during follow up with lung being the most common site.
Ten patients (71.4%) died of disease (DOD). The median survival of patients who did not receive chemotherapy (2 to 31 months) was almost similar to those who did receive chemotherapy (3 to 8 months). Two patients remained alive and disease free at 16 (case 2) and 11 months (case 11) respectively. One patient died of pneumonia two months after surgery (case 13). The follow up was not mentioned in one case (case 12).
Due to the rarity of renal SCC, definitive therapeutic protocols are missing. However, existing information suggests that survival following surgery for renal SCC is poor, and consideration of neoadjuvant chemotherapy may be merited. Renal pelvis SCC is an aggressive tumor with a median survival of 8.2 months. A significant improvement in overall survival has been reported with the use of platinum-based chemotherapy (20 months versus 8 months, p=0.02) for cases of renal SCC. In addition favorable outcomes have also been seen with cisplatin-based chemotherapy for small cell carcinoma arising from non renal genitourinary and other extra pulmonary sites [3]. Galanis et al. observed a 72% response rate in 22 patients with extra pulmonary small cell carcinoma treated with platinum-based chemotherapy regimen [17]. Regimens containing doxorubicin had a 57% response rate with a median duration of response of only 4.5 months. Lo et al. reported an overall response rate of 69% to combination chemotherapy with cisplatin and etoposide in 13 patients with extrapulmonary small cell carcinoma.
Conclusion
Small cell carcinoma of the renal pelvis is a rare and aggressive neoplasm with median survival of less than a year. The poor survival is due to a high incidence of occult metastasis at the time of presentation. Even though definitive treatment protocols are lacking, the use of chemotherapy along with surgery can promote limited survival.
Authors' Contribution
PPT: prepared the draft manuscript
DK
: did the literature search and helped in preparation of manuscript
AS
: helped in preparation of manuscript
SS
: Editing of the manuscript
HB
: Preparation of final manuscript
MJS
: Overall supervision and editing of manuscript
Conflict of Interests
The authors state that they have no conflict of interest(s).
Ethical Considerations
Written informed consent was obtained from the patient for publication of this case report
Funding
None Declared
Acknowledgement
The authors wish to express their thanks to Dr. S.N. Shukla, Honorary Director. Dr. R.K. Vyas and Dr. Kiran Kothari, Deputy Directors of the Gujarat cancer and research institute to allow us to publish this scientific data.
References
[1]. Remick SC, Ruckdeschel JC. Extrapulmonary and pulmonary small-cell carcinoma: tumor biology, therapy, and outcome. Medical and Pediatric Oncology 1992; 20 (2):89–99. [Pubmed].
[2]. Haider K, Shahid RK, Finch D, et al. Extrapulmonary small cell cancer: a Canadian province's experience. Cancer 2006; 107 (9):2262–2269. [Pubmed].
[3]. Majhail NS, Elson P, Bukowski RM. Therapy and outcome of small cell carcinoma of the kidney: report of two cases and a systematic review of the literature. Cancer 2003; 97(6):1436–1441. [Pubmed].
[4]. La Rosa S, Bernasconi B, Micello D, Finzi G, Capella C. Primary small cell neuroendocrine carcinoma of the kidney: morphological, immunohistochemical, ultrastructural and cytogenetic study of a case and review of the literature. Endocrine Pathology 2009; 20(1):24–34. [Pubmed].
[5]. Mills SE, Wolfe JT, Weiss MA et al,. Small cell undifferentiated carcinoma of the urinary bladder: a light-microscopic, immunocytochemical, and ultrastructural study of 12 cases. Am J Surg Path 1987; 11 (8): 606–617. [Pubmed].
[6]. Essenfeld H, Manivel JC, Benedetto P, Albores-Saavedra J. Small cell carcinoma of the renal pelvis: a clinicopathological, morphological and immunohistochemical study of 2 cases. J of Urol 1990; 144 (2): 344–347. [Pubmed].
[7]. Guillou L, Duvoisin B, Chobaz C, Chapuis G, Costa J. Combined small-cell and transitional cell carcinoma of the renal pelvis: a light microscopic, immunohistochemical, and ultrastructural study of a case with literature review. Archives of Pathology and Laboratory Medicine 1993; 117 (3): 239–243. [Pubmed].
[8]. Mazzucchelli L, Studer UE, Kraft R. Small-cell undifferentiated carcinoma of the renal pelvis 26 years after subdiaphragmatic irradiation for non-Hodgkin's lymphoma. British Journal of Urology 1995; 76 (3):403–404. [Pubmed].
[9]. Kuromatsu I, Hayashi N, Yanagawa M, Tochigi H, Kawamura J. Combined small cell and transitional cell carcinoma of renal pelvis: a case report. Hinyokika Kiyo 1995; 41 (1):47–50. [Pubmed].
[10]. Kitamura M, Miyanaga T, Hamada M, Nakata Y, Satoh Y, Terakawa T. Small cell carcinoma of the kidney: case report. International Journal of Urology 1997; 4 (4):422–424. [Pubmed].
[11]. Kojima S, Mine M, Sekine H. Small cell carcinoma of the kidney. A case report. Nippon Hinyokika Gakkai Zasshi 1998; 89 (6): 614–617. [Pubmed].
[12]. Chuang CK, Liao SK. A retrospective immunohistochemical and clinicopathological study of small cell carcinomas of the urinary tract. Chang Gung Medical Journal 2003; 26 (1):26–33. [Pubmed].
[13]. Shimasaki N, Inoue K, Nishigawa H, Kuroda N, Shuin T. Combined small cell carcinoma and sarcomatoid squamous cell carcinoma in the renal pelvis. International Journal of Urology 2005; 12 (7):686–689. [Pubmed].
[14]. Banerji J, Korula A, Panicker J. Multicentric small cell neuroendocrine neoplasm of the renal pelvis and ureter with concomitant focal high-grade urothelial carcinoma of the ureter: a case report. Indian Journal of Urology 2008; 24 (4):571–574. [Pubmed].
[15]. Patil S, kaza RCM, kakkar AK, Chamberlain RS. Small cell carcinoma of the renal pelvis: A case report and review of literature. ISRN Urology 2011;2011: 786505. [Pubmed].
[16]. Senecal L. Undifferentiated small cell carcinoma of the renal pelvis with massive invasion of the kidney. Union Med Can 1985; 114 (2): 147. [Pubmed].
[17]. Galanis E, Frytak S, Lloyd RV. Extrapulmonary small cell carcinoma. Cancer 1997; 79 (9):1729–1736. [Pubmed].
[18]. Lo RG, Canzonieri V, Veronesi A et al. Extrapulmonary small cell carcinoma: a single-institution experience and review of the literature. Annals of Oncology 1994; 5 (10):909–913. 1994. [Pubmed].

