Case Report

Pancreatic Metastasis of Merkel Cell Carcinoma Diagnosed by EUS-FNA: A Case Report and Review of Literature.
1 Wuttiporn Manatsathit 2Randy Tashjian3 Baljinder Gill,4, Pornchai Leelasinjaroen5Paul Mazzara
- 1Internal Medicine, St John Hospital and Medical Center, Michigan, St Clair Shores, United States
- Monday, September 16, 2013
- Friday, September 20, 2013
- Sunday, January 26, 2014
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ((http://creativecommons.org/licenses/by/3.0)which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Merkel cell carcinoma (MCC) is a rare primary skin tumor with aggressive behavior. We report a case of metastatic Merkel cell carcinoma of the pancreas diagnosed by endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) in an Asian male and review of the literature
Case presentation
a 65-year-old Filipino man with history of primary cutaneous MCC of the left forearm presented with intractable nausea and vomiting. The patient was found to have a 3.5 x 4 cm pancreatic mass by abdominal computerized tomography. EUS-FNA was subsequently performed. The cytological and pathological examination revealed atypical cells with hyperchromatic nuclei. The immunohistochemical stains were consistent with MCC. To our knowledge, only seven such cases have been reported in the English literature.
Discussion
EUS-FNA of the pancreas can effectively obtain tissue adequate for establishing the diagnosis of metastatic MCC. Thorough knowledge of clinical history and accurate interpretation of cytology and cell block preparation obtained by EUS-FNA are essential to establish the definite diagnosis of MCC. Patients with metastatic MCC to the pancreas have a grave.
Keywords
Merkel cell carcinoma; pancreas; pancreatic mass; pancreatic tumor; neuroendocrine carcinoma
Introduction
Merkel cell carcinoma (MCC) is a rare aggressive primary neuroendocrine neoplasm of skin and/or subcutaneous soft tissues. It was first described in 1972 by Toker as a trabecular carcinoma and was subsequently identified as a neoplasm of cutaneous mechanoreceptor cells - Merkel cells.{1]The incidence of the disease is estimated to be less than 0.5 per 100,000 person-years.[4]Typically, the tumor presents as a non-tender, rapidly growing, glistening, violaceous papule or nodule found in sun-exposed areas of elderly Caucasians.{5,8] The mean age at the time of diagnosis is between sixty and seventy years of age,{5,9] but younger patients have been known to develop MCC as well.[10,13]The most common location is the head and neck region, followed by the extremities and the trunk.[5]Most of MCC is caused by Merkel cell polyomavirus. This explains why immunocompromised individuals appear to be more susceptible to developing MCC. For instance, patients undergoing solid organ transplantation have ten-fold increase in risk of MCC whereas patients suffering from human immunodeficiency virus (HIV) infections have a 14-fold increase in risk of MCC.[14,16]
Histologically, MCC is similar to other small blue cell neoplasms such as small cell carcinoma of lung, cutaneous large cell lymphoma, neuroblastoma, metastatic carcinoid tumor, and Langerhans cell histiocytosis. While it may be fairly well circumscribed, MCC more often exhibits an infiltrative growth pattern into the surrounding tissues under microscopy. A trabecular or “organoid” growth pattern is sometimes observed, but most cases of MCC exhibit neoplastic cells arranged in solid sheets. Neoplastic cells characteristically possess minimal cytoplasm and nuclear size ranging in size from intermediate to large, with the intermediate cell type being the most common. The chromatin of the neoplastic cells typically has a neuroendocrine appearance, that is, finely granular (“salt and pepper”). Molding of nuclei and nuclear crush artifact are often present, and mitotic figures and single cell apoptosis are frequently encountered.
The neoplastic cells that compose MCC are virtually always immunoreactive with neuroendocrine immunohistochemical markers, such as chromogranin, synaptophysin, and CD56 (neural cell adhesion molecule [N-CAM]). Most MCCs also express epithelial markers, chiefly Cam 5.2, pancytokeratin (CK AE1/AE3), cytokeratin 20 (CK 20), and epithelial membrane antigen (EMA). A paranuclear or “dot-like” pattern of staining may be seen with cytokeratin stains– a very useful finding in establishing a diagnosis of MCC. Variable positivity for CD99 and CD117 has been observed. Generally, MCC is not immunoreactive with lymphoid markers (e.g., CD45/leukocyte common antigen [LCA]), melanoma markers (e.g., melan-A/mart-1, HMB-45, and S-100 protein), or thyroid transcription factor-1 (TTF-1). The latter is often positive in small cell neuroendcrine carcinoma of the lung, a tumor with very similar histology.
The morphology of cells is similar in cytologic smears and cell block preparations from material obtained by fine-needle aspiration (FNA). Small, cohesive clusters of neoplastic cells with scant cytoplasm and high nuclear to cytoplasmic ratios are typically observed. Other features include hyperchromatic nuclei exhibiting nuclear molding and finely granular nuclear chromatin. If a cell block preparation is available, a panel of immunohistochemical stains may be performed to confirm a suspected diagnosis, and the staining pattern would be similar to that observed in tissue specimens
Case Presentation
A 65-year-old Filipino male presented with chronic epigastric pain of four-month duration and intractable nausea and vomiting for a period of one day. Two years prior to presentation, in 2008, the patient had returned to United States from the Philippines after undergoing surgical excision of a left forearm mass. Surgery was followed by split thickness skin grafting. The initial pathology report of the excisional biopsy performed in the Philippines was diagnosed as a malignant small round cell neoplasm. Because of the unclear diagnosis, a second biopsy was performed once the patient returned to the United States, which revealed granulation tissue without an evidence of neoplasia.
Microscopic examination of a third excisional biopsy performed three weeks later proved positive for a malignant neoplasm. Neoplastic cells were noted to infiltrate into the surrounding fibroadipose tissue in a sheet-like arrangement (Figure I)
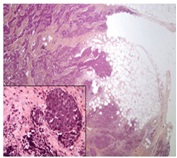
Figure 1: Skin Biopsy (20x). The Images Show Nests And Solid Sheets Of Neoplastic Cells Infiltrating Into The Surrounding Fibroadipose Tissue. The Neoplastic Cells Also Contained Large Hyperchromatic Nuclei And Finely Granular Nuclear Chromatin.
Their nuclei were large, hyperchromatic, and contained finely granular nuclear chromatin. The neoplastic cells were immunoreactive with chromogranin, synaptophysin, and cytokeratin 20 (CK 20). CK 20 stained positively in a paranuclear “dot-like” pattern (Figure 2C) the neoplastic cells did not stain with cytokeratin 7 (CK 7), thyroid transcription factor-1 (TTF-1), S-100 protein, or melan-A/mart1. The histopathologic features and immunohistochemical-staining pattern were felt to be diagnostic for primary cutaneous MCC. Accordingly, the patient underwent external-beam radiation and was treated with chemotherapy based on the regimen for small cell carcinoma of lung.
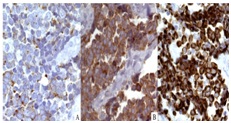
Figure 2: Immunohistochemical stains of skin biopsy (40X). The neoplastic cells were immunoreactive with chromogranin (A), synaptophysin (B), and cytokeratin 20 (CK 20) (C). The specimen was negative for CK7, (TTF-1), and S-100 protein.
Two years after initial presentation, in 2010, the patient presented to the emergency department (ED) complaining of chronic epigastric pain and intractable nausea and vomiting. He was afebrile with stable vital signs. Physical examination of the abdomen was negative; no distention or tenderness was appreciated. The remainder of physical examination was unremarkable. Laboratory tests revealed no significant abnormalities except for mild anemia (hemoglobin 10.5 g/dL). The patient’s complete blood count and basic metabolic panel were as follows: white blood cell count 11,300/µl, platelet count 181,000/µl, sodium 141 mmol/L, potassium 3.9 mmol/L, chloride 102 mmol/L, bicarbonate 25 mmol/L, calcium 8.3 mg/dL, blood urea nitrogen (BUN) 14 mg/dL, and creatinine 0.54 mg/dL. Liver function tests were within reference range: total bilirubin 0.9 mg/dL, alkaline phosphatase 61 units/L, AST 25 unit/L, ALT 11 unit/L, albumin 3.1 gm/dL, and globulin 2.4gm/dL. Carbohydrate antigen 19-9 (CA 19-9) was 1.9 units/ml and carcinoembryonic antigen (CEA) was 2.7 ng/ml.
In the ED, abdominal ultrasonography was performed, which
revealed a solid, hypoechoic mass in the head of the pancreas and uncinate process. Computerized tomography (CT) of the abdomen subsequently showed a 3.5 x 4.0 cm complex, predominantly cystic mass involving the pancreatic head and uncinate process. An endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of the pancreatic mass was performed for definite diagnosis. The EUS-FNA specimen was evaluated by performing cytology smears and cell block preparation.
The cytological examination revealed rare cohesive fragments of benign enteric epithelium admixed with numerous clusters of cohesive atypical cells with scant cytoplasm and hyperchromatic nuclei (Figure 3). A cell block preparation also revealed neoplastic cells with hyperchromatic nuclei and finely granular nuclear chromatin (Figure 4).
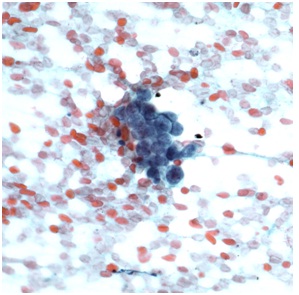
Figure 3: Cytological examination of the EUS-FNA material (40X). The image shows a cluster of malignant neuroendocrine cells with high nuclear to cytoplasmic ratio.
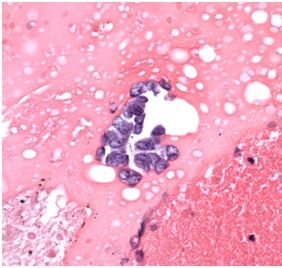
Figure 4: Hematoxylin and eosin stain from cell block preparation (40X). The neoplastic cells were small to intermediate in size, possessed hyperchromatic nuclei that exhibited prominent nuclear molding, and contained finely granular nuclear chromatin, as seen here on the cell block preparation from the cytologic material obtained by FNA.
The neoplastic cells were immunoreactive with chromogranin, synaptophysin, and CK 20 in a paranuclear “dot-like” pattern (Figure 5). The immunohistochemical stains were negative for TTF-1, S-100, and LCA. Considering previous history of MCC and the immunohistochemical staining pattern, a diagnosis of metastatic MCC was rendered. The patient was treated with local external-beam radiation and concurrent chemotherapy. Multiple distant lymph nodes metastasis was subsequently discovered, and the patient ultimately expired seven months after the diagnosis was made.
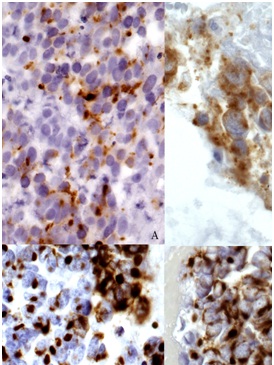
Figure 5: Hematoxylin and eosin(A) and immunohistochemical stains from cell block preparation (40X). The neoplastic cells expressed chromogranin (B), synaptophysin (C), and cytokeratin AE1/AE3 (CK AE1/AE3) (D). The specimen was also negative for CK7, (TTF-1), and S-100 protein.
Discussion
Pancreas is an elongated retroperitoneal organ situated horizontally and parallel to splenic vein posteriorly. It is also encased by the stomach anteriorly and duodenum surrounding the head of the pancreas. Considering this unique anatomical relationship, tissue acquisition of pancreas is almost impossible without direct visualization. Since the introduction of imaging-guided biopsy (e.g. CT, US, and EUS), pancreatic biopsy can be safely performed. A randomized crossover trial comparing EUS-FNA to percutaneous CT/US guided biopsy of pancreas demonstrated no significant differences in accuracy between the two methods.[17,18]However, EUS-FNA contains lower risk of needle-tract seeding and ability to detect small lesion undetectable from CT scan or magnetic resonance imaging (MRI).[19,20]Additionally, EUS-FNA is more cost effective and vascular structure can be avoided during biopsy by using color Doppler. EUS-FNA, however, requires steep learning curve and constant exposure.[21]Following tissue acquisition, specimen can be prepared by smearing the material on a slide for cytological examination or fixing in formalin for histological examination. Regardless of tissue preparation method, EUS-FNA has been proved to be effective in the diagnosis of pancreatic lesions with sensitivity of 75 to 95 percent and specificity of 88 to 100 percent.[22,23] Only a few studies, however, compared these two methods. Kopelman et al. reported the higher sensitivity of EUS-FNA cytology whereas cell block was found to be more specific for the diagnosis of pancreatic mass.[24].Noda et al. performed a prospective study comparing the efficacy of these two methods from specimen obtained by EUS-FNA. The results demonstrated that cell block had higher sensitivity and higher accuracy.[25]
These inconsistencies among studies, however, can be explained by variety of tissue preparation methods, type of specimens, and proficiency of pathologist in each facility. Overall, cytology and cell block are complementary and, therefore, should be utilized to achieve the highest accuracy.
MCC is an aggressive neuroendocrine neoplasm of the skin and subcutis. At presentation, seven percent of patients already suffer from distance metastases.[9].MCC has the potential to metastasize to any site with distance skin as the most common followed by regional lymph node, liver, bone, and brain, but pancreatic involvement is exceedingly rare.[26,27].According to Adsay et al., not a single case of MCC was observed in 4,955 pancreatic specimens obtained during autopsy, and only one out of 973 surgical pancreatic resection specimens was positive for MCC.[28] Since MCC was first described, only seven prior cases of metastatic MCC to the pancreas have been reported in the English literature.[28,34]
The characteristic and details of each case are shown in (table 1).
| Case |
Age/Sex |
Time of diagnosis after primary lesion (months) |
Clinical Presentation |
Location in Pancreas |
Cystic Mass |
Survival Time (months) |
| Adsay et al. [19] |
Not reported |
Not reported |
Not reported |
Tail |
Yes |
Not reported |
| Dim et al.* [20] |
79/F |
15 |
Abdominal pain |
Tail |
No |
Not reported |
| Ouellette et al. [21] |
64/M |
4 |
Jaundice |
Head |
No |
19 |
| Bernstein et al. [22] |
56/M |
5 |
Asymptomatic |
Tail |
No |
Not reported |
| Bachmeyer et al. [23] |
57/M |
5 |
Jaundice |
Body |
Yes |
2 |
| Krejci et al. [24] |
62/M |
4 |
Abdominal mass |
Head |
No |
5 |
| Bachmann et al. [25] |
82/F |
12 |
Abdominal mass |
Body and tail |
No |
Not reported |
| Current case |
65/M |
24 |
v |
Head |
Yes |
7 |
Six of the patients, including the patient presented in this case report were male, and two were female. The majority of the patients (75%) developed metastasis to the pancreas within two years of initial diagnosis. There does not appear to be a definite predilection site for pancreatic metastasis, as the head, body, and tail of pancreas were roughly equally involved. The most common clinical presentation was an abdominal mass, while obstructive jaundice occurred in two out of eight cases. One patient was asymptomatic on initial presentation. CA 19-9 was found to be slightly elevated in one patient, with the value of 94.4 IU/L (normal, 0-37 IU/L).[] Imaging studies, either by abdominal CT or EUS, showed a predominantly cystic mass in three out of eight patients. EUS-FNA was performed in three cases in order to obtain cytological material for diagnosis. Follow-up information was available for only three of the reported cases. Three patients, including the patient presented in this case report, died within one year of a diagnosis of pancreatic metastasis. One patient survived for 19 months after undergoing a pancreaticoduodenectomy (Whipple procedure).
Generally, patients with metastatic MCC have a dismal prognosis. The reported overall five-year survival rate associated with metastatic MCC ranges from 40% to 60%, and disease specific death is approximately 30%.[35,37] Moreover, according to Fields et al., the five-year survival associated with distant metastases is estimated to be roughly 18%.[4] As our patient and four reported cases deceased within five years of the diagnosis, the prognosis of MCC with pancreatic metastasis is extremely poor.
Conclusion
In summary, cytological preparation both smears and cell block preparation along with immunohistochemical stains play a crucial role in diagnosing MCC. EUS-FNA seems to effectively provide adequate pathological specimen for the diagnosis. Gastroenterologists and pathologists should continue to consider metastatic MCC in the differential diagnosis of a pancreatic mass in a patient diagnosed with MCC within two-year duration.
Authors' Contribution
WM and BG carried out the literature review and prepared the manuscript, RT performed review of the pathological specimens & histological stains and prepared the pathology description, PL assisted in reviewing of the literature and , PM supervised the preparation of manuscript and reviewed the manuscript. It is important to state that all authors read and approved the final manuscript for submission
Conflict of Interests
The authors declare that there are no conflict of interests.
Ethical Considerations
Written informed consent was taken from the patient for publication of this case report.
Funding
None declared
Acknowledgement
None
References
[1]Toker C. Trabecular Carcinoma Of The Skin. Archives Of Dermatology 1972;105:107-10.[pubmed]
[2]Tang CK, Toker C. Trabecular Carcinoma Of The Skin: An Ultrastructural Study. Cancer 1978;42:2311-21.[[pubmed]
[3]Tang CK, Toker C. Trabecular Carcinoma Of The Skin: Further Clinicopathologic And Ultrastructural Study. The Mount Sinai Journal Of Medicine, New York 1979;46:516-23.[pubmed]
[4]Fields RC, Busam KJ, Chou JF, Et Al. Five Hundred Patients With Merkel Cell Carcinoma Evaluated At A Single Institution. Annals Of Surgery 2011;254:465-73; Discussion 73-5.[[pubmed]
[5]Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel Cell Carcinoma Demographics, Morphology, And Survival Based On 3870 Cases: A Population Based Study. Journal Of Cutaneous Pathology 2010;37:20-7.
[6]Brissett AE, Olsen KD, Kasperbauer JL, Et Al. Merkel Cell Carcinoma Of The Head And Neck: A Retrospective Case Series. Head & Neck 2002;24:982-8.[[pubmed]
[7]Gollard R, Weber R, Kosty MP, Greenway HT, Massullo V, Humberson C. Merkel Cell Carcinoma: Review Of 22 Cases With Surgical, Pathologic, And Therapeutic Considerations. Cancer 2000;88:1842-51.[[pubmed]
[8].Pectasides D, Pectasides M, Economopoulos T. Merkel Cell Cancer Of The Skin. Annals Of Oncology : Official Journal Of The European Society For Medical Oncology / ESMO 2006;17:1489-95.[pubmed]
[9]Lemos BD, Storer BE, Iyer JG, Et Al. Pathologic Nodal Evaluation Improves Prognostic Accuracy In Merkel Cell Carcinoma: Analysis Of 5823 Cases As The Basis Of The First Consensus Staging System. Journal Of The American Academy Of Dermatology 2010;63:751-61.
[10]. Kukko H, Vuola J, Suominen S, Koljonen V. Merkel Cell Carcinoma In A Young Pregnant Woman. Journal Of Plastic, Reconstructive & Aesthetic Surgery : JPRAS 2008;61:1530-3.[[pubmed]
[11].Rodriguez-Prieto MA, Alonso-Alonso T, Toribio-Garcia JA, Mateos-Hernandez A. [Young Patient With Eyelid Merkel Carcinoma: Mohs Microsurgery Versus Exenteration]. Arch Soc Esp Oftalmol 2009;84:581-4[pubmed]
[12]Moya CE, Guarda LA, Dyer GA, Silva EG, Shah S. Neuroendocrine Carcinoma Of The Skin In A Young Adult. American Journal Of Clinical Pathology 1982;78:783-5.[pubmed]
[13]Diallo M, Diallo KB, Niang A, Et Al. [Merkel Cell Carcinoma Of The Gingival Mucosa In A Black Young Adult]. Rev Laryngol Otol Rhinol (Bord) 2011;132:111-4.[pubmed]
[14]. Penn I, First MR. Merkel's Cell Carcinoma In Organ Recipients: Report Of 41 Cases. Transplantation 1999;68:1717-21[pubmed]
[15]Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel Cell Carcinoma And HIV Infection. Lancet 2002;359:4978[pubmed]
[16]Lanoy E, Costagliola D, Engels EA. Skin Cancers Associated With HIV Infection And Solid-Organ Transplantation Among Elderly Adults. Int J Cancer 2010;126:1724-31.[[pubmed]
[17]Horwhat JD, Paulson EK, Mcgrath K, Et Al. A Randomized Comparison Of EUS-Guided FNA Versus CT Or US-Guided FNA For The Evaluation Of Pancreatic Mass Lesions. Gastrointestinal Endoscopy 2006;63:966-75.[[pubmed]
[18]Erturk SM, Mortelé KJ, Tuncali K, Saltzman JR, Lao R, Silverman SG. Fine-Needle Aspiration Biopsy Of Solid Pancreatic Masses: Comparison Of CT And Endoscopic Sonography Guidance. American Journal Of Roentgenology 2006;187:1531-5.[[pubmed]
[19]Chen VK, Arguedas MR, Kilgore ML, Eloubeidi MA. A Cost-Minimization Analysis Of Alternative Strategies In Diagnosing Pancreatic Cancer. The American Journal Of Gastroenterology 2004;99:2223-34.[[pubmed]
[20]Micames C, Jowell PS, White R, Et Al. Lower Frequency Of Peritoneal Carcinomatosis In Patients With Pancreatic Cancer Diagnosed By EUS-Guided FNA Vs. Percutaneous FNA. Gastrointestinal Endoscopy 2003;58:690-5.[[pubmed]
[21]Eloubeidi MA, Tamhane A. EUS-Guided FNA Of Solid Pancreatic Masses: A Learning Curve With 300 Consecutive Procedures. Gastrointestinal Endoscopy 2005;61:700-8.[[pubmed]
[22]Eloubeidi MA, Chen VK, Eltoum IA, Et Al. Endoscopic Ultrasound–Guided Fine Needle Aspiration Biopsy Of Patients With Suspected Pancreatic Cancer: Diagnostic Accuracy And Acute And 30-Day Complications. The American Journal Of Gastroenterology 2003;98:2663-8.[pubmed]
[23]Voss M, Hammel P, Molas G, Et Al. Value Of Endoscopic Ultrasound Guided Fine Needle Aspiration Biopsy In The Diagnosis Of Solid Pancreatic Masses. Gut 2000;46:244-9.[[pubmed]
[24]Kopelman Y, Marmor S, Ashkenazi I, Fireman Z. Value Of EUS‐FNA Cytological Preparations Compared With Cell Block Sections In The Diagnosis Of Pancreatic Solid Tumours. Cytopathology 2011;22:174-8.[[pubmed]
[25]Noda Y, Fujita N, Kobayashi G, Et Al. Diagnostic Efficacy Of The Cell Block Method In Comparison With Smear Cytology Of Tissue Samples Obtained By Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Journal Of Gastroenterology 2010;45:868-75.[pubmed]
[26]Voog E, Biron P, Martin JP, Blay JY. Chemotherapy For Patients With Locally Advanced Or Metastatic Merkel Cell Carcinoma. Cancer 1999;85:2589-95.
[27]Medina-Franco H, Urist MM, Fiveash J, Heslin MJ, Bland KI, Beenken SW. Multimodality Treatment Of Merkel Cell Carcinoma: Case Series And Literature Review Of 1024 Cases. Annals Of Surgical Oncology 2001;8:204-8[pubmed]
[28]Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary Tumors Of The Pancreas: An Analysis Of A Surgical And Autopsy Database And Review Of The Literature. Virchows Archiv : An International Journal Of Pathology 2004;444:527-35.[[pubmed]
[29]Dim DC, Nugent SL, Darwin P, Peng HQ. Metastatic Merkel Cell Carcinoma Of The Pancreas Mimicking Primary Pancreatic Endocrine Tumor Diagnosed By Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology: A Case Report. Acta Cytologica 2009;53:223-8[pubmed]
[30]Ouellette JR, Woodyard L, Toth L, Termuhlen PM. Merkel Cell Carcinoma Metastatic To The Head Of The Pancreas. JOP : Journal Of The Pancreas 2004;5:92-6[pubmed]
[31]Bernstein J, Adeniran AJ, Cai G, Et Al. Endoscopic Ultrasound-Guided Fine-Needle Aspiration Diagnosis Of Merkel Cell Carcinoma Metastatic To The Pancreas. Diagnostic Cytopathology 2012.[[pubmed]
[32]Bachmeyer C, Alovor G, Chatelain D, Et Al. Cystic Metastasis Of The Pancreas Indicating Relapse Of Merkel Cell Carcinoma. Pancreas 2002;24:103-5[pubmed]
[33]Krejci K, Tichy T, Horak P, Et Al. Merkel Cell Carcinoma Of The Gluteal Region With Ipsilateral Metastasis Into The Pancreatic Graft Of A Patient After Combined Kidney-Pancreas Transplantation. Onkologie 2010;33:520-4.[[pubmed]
[34]. Bachmann J, Kleeff J, Bergmann F, Et Al. Pancreatic Metastasis Of Merkel Cell Carcinoma And Concomitant Insulinoma: Case Report And Literature Review. World Journal Of Surgical Oncology 2005;3:58.[[pubmed]
[35]O'Connor WJ, Roenigk RK, Brodland DG. Merkel Cell Carcinoma. Comparison Of Mohs Micrographic Surgery And Wide Excision In Eighty-Six Patients. Dermatologic Surgery : Official Publication For American Society For Dermatologic Surgery [Et Al] 1997;23:929-33.[pubmed]
[36]Ratner D, Nelson BR, Brown MD, Johnson TM. Merkel Cell Carcinoma. Journal Of The American Academy Of Dermatology 1993;29:143-56.[pubmed]
[37]Skelton HG, Smith KJ, Hitchcock CL, Mccarthy WF, Lupton GP, Graham JH. Merkel Cell Carcinoma: Analysis Of Clinical, Histologic, And Immunohistologic Features Of 132 Cases With Relation To Survival. Journal Of The American Academy Of Dermatology 1997;37:734-9[pubmed]

