Case Report

Metastatic Clear Cell Renal Cell Carcinoma: A Mimicker of Primary Clear Cell Carcinoma Ovary: How
to Differentiate?
*,Monisha Choudhury *Deepti Verma, *Alka Mittal, *,Shveta Wadhwa *Shaji Thomas
- *Pathology, Lady Hardinge Medical College, Delhi, , India
- Submitted Tuesday, April 29, 2014
- Accepted:Thursday, May 22, 2014
- Published Thursday, September 04, 2014
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Renal cell carcinomas account for about 3/ 4th of the renal tumors. About 1 /3rd of these have metastatic disease. However, metastasis to ovaries is rare. Only few cases have been reported in literature. We are reporting a case of 45 year old lady presenting with simultaneous clear cell renal cell carcinoma along with ovarian metastasis.
Case presentation
A 45 year old lady presented with complaints of hematuria for 7 months. A diffuse lump was noticed in the right lumbar region and iliac fossa measuring 10 X 8 cm in size. CECT of abdomen showed a heterogeneously enhancing lesion measuring 9.9 X 8.4 X 8.2 cm in upper and midpole of right kidney suggestive of oncocytoma or renal cell carcinoma (RCC) and a heterogeneously enhancing right adnexal lesion likely to be of malignant etiology. However, serum CA-125 level was within normal limits. The right sided radical nephrectomy was performed along with total abdominal hysterectomy with bilateral salpingoophrectomy. A diagnosis of right sided renal cell carcinoma with metastasis to right ovary was established on histopathological and immunohistochemical examination.
Conclusion
The metastatic clear cell RCC should be considered as a differential diagnosis of clear cell tumors in ovary. The distinction is important for their therapeutic and prognostic implications. This is possible on the basis of their histomorphological and immunohistochemical characteristics.
Keywords
renal cell carcinoma, clear cell carcinoma, ovarian metastasis
Introduction
Renal cell carcinoma (RCC) is a common malignant solid tumor accounting for 75% of renal neoplasms and 3% of all adult malignancies [1]. About one-third of all patients with a de novo diagnosis of renal carcinoma have metastatic disease [2]. Most common metastatic sites in order of frequency are the lungs, bones, liver, lymph nodes, adrenals and brain [3]. Metastasis to ovary is rare. Only 23 cases have been reported in literature so far [4]. In most cases, the ovarian mass was detected after the diagnosis of the renal tumor [3]. However, our case presented as a clear cell RCC with simultaneous ovarian metastasis.
Case Presentation
A 45 year old lady presented to surgery outpatient department with complaints of hematuria for 7 months. She had five such episodes during this period. Abdominal examination revealed a diffuse lump in the right lumbar region and iliac fossa measuring 10 X 8 cm, not moving with respiration.
Contrast Enhanced CT scan of abdomen showed a heterogeneously enhancing lesion with central nonenhancing area (stellate scar) measuring 9.9 X 8.4 X 8.2 cm in upper and midpole of right kidney suggestive of oncocytoma or RCC. Right ovary showed heterogeneously enhancing adnexal lesion likely to be of malignant etiology. Serum CA-125 level was normal.
The right sided radical nephrectomy was performed along with total abdominal hysterectomy with bilateral salpingoophrectomy.
The intra operative peritoneal washing performed was negative for the malignant
cells. The kidney weighed 480g and measured 13 X 9 X 9 cm. The external surface was bosselated; cut surface showed a well circumscribed partly encapsulated solid mass measuring 6.5 X 6 X 7.5 cm occupying the upper pole and mid portion of kidney with areas of hemorrhage and fibrosis. Tumor was also seen extending beyond the renal capsule. The Gerota’s fascia and perinephric fat were free of tumor. Four nodules were identified varying in size from 0.5 to 1 cm in diameter; one of which occupied inferior pole of the kidney. Right sided ovarian mass measured 12 X 8 X 8 cm; it was encapsulated, bosselated with no external breach present externally. The cut surface was solid cystic with cysts ranging in size from 0.3 to 2.5 cm in diameter. The serosangineous fluid was drained out from many of the cysts while few contained mucoid material. Uterus with cervix was unremarkable except for a subserosal fibroid measuring 0.6 cm in right lateral uterine wall. The left ovary was unremarkable.
Microscopically, the renal and ovarian tumor tissue showed similar morphology (figure 1). The tumor tissue was arranged in alveolar pattern with nests being separated by fibrovascular septae. The individual tumor cells were large sharply outlined, had clear abundant cytoplasm, central round nuclei showing moderate anisonucleosis, fine chromatin and 0-1 prominent nucleoli. Foci of calcification and fibrosis were also seen. The tumor extended beyond the renal capsule but Gerota’s fascia and perinephric fat were uninvolved.
No evidence of vascular invasion was found in the sections studied from kidney
and the ovaries. The tumor cells were PAS positive with sensitivity to diastase. Immunohistochemistry showed positivity for Pan-CK, vimentin and CD10 in both ovarian and renal tumor tissue (figure 2)and(figure 3) and negativity for CK7 and CA-125. The diagnosis of multifocal RCC (clear cell type), right kidney, Fuhrman nuclear grade 2 with extension beyond renal capsule and metastasis to right ovary was thus made.
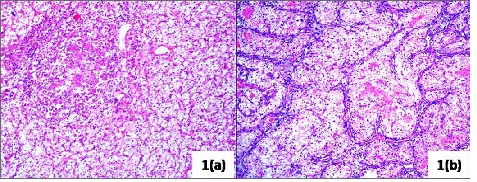
Figure 1: The renal and ovarian tumor tissue showed similar morphology. The tumor tissue was arranged in alveolar pattern with nests being separated by fibrovascular septae. The individual tumor cells were large sharply outlined, had clear abundant cytoplasm, central round nuclei showing moderate anisonucleosis, fine chromatin and 0-1 prominent nucleoli. 1(a): the renal tumor (H/E, 10X); 1(b): the ovarian tumor (H/E, 10X).
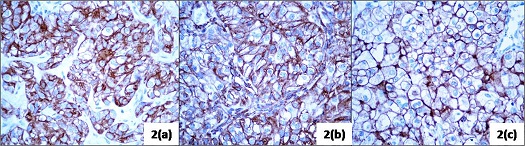
Figure 2: Immunohistochemistry of the renal tumor (40X): (a) positive for Pan CK (b) positive for vimentin (c) positive for CD 10
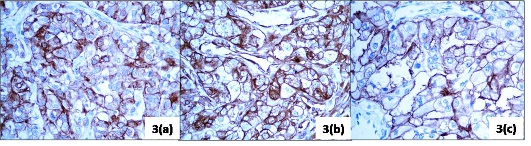
Figure 3: Immunohistochemistry of the ovarian tumor (40X): (a) positive for Pan CK (b) positive for vimentin (c) positive for CD 10
Discussion
The RCC more commonly metastasizes via lymphatic and venous routes spreading to lungs (50-60%), lymph nodes (36%), bones (30-40%), liver (30-40%) and brain (5%) [1]. Metastasis to unusual sites like skin is also known. However, ovarian metastasis is rare. Metastatic ovarian carcinomas most commonly originate from carcinoma of stomach, colon, breast and lymphoma [1]. Spread to ovaries is thought to occur by retrograde venous embolism through renal veins to the ovarian vessels. Two-third reported cases result from left sided lesion as left sided ovarian vein drains directly into left renal vein. The exact reason of rarity of ovarian metastasis remains unclear. It is suggested that RCC affects males more commonly than females, during 6th and 7th decades. By this time ovaries become fibrotic and atrophic and blood flow is reduced. Therefore, fewer emboli would be carried to ovary after menopause than to larger and more vascular organs. Furthermore, vascular sclerosis of ovary would reduce the clump of tumor cells getting through the arterioles into a more suitable environment of the capillary beds or into thin walled veins [5].Distinction of the metastatic clear cell RCC from the primary clear cell carcinoma of ovary is difficult but certain histopathological features and immunohistochemistry can be of great help.
Primary clear cell carcinoma or mesonephroid adenocarcinoma of ovary occurs between 40 and 60 years of age. These account for less than 5% of the ovarian malignancies and range upto 30 cm in diameter. The cut surface shows thick walled unilocular cysts with multiple yellow-beige fleshy nodules protruding into the lumen, or a multiloculated cystic mass with cysts containing watery or mucinous fluid. Microscopically, many patterns are seen; solid and tubulopapillary being more common. Solid pattern is characterized by sheets of polyhedral cells with abundant clear glycogen rich cytoplasm separated by delicate fibrovascular septae or dense hyalinized fibrotic stroma. Tubulopapillary pattern shows papillae with variable complexity and often hyalinized papillary cores. Hyalinized and homogenous eosinophilic stromal fibrosis is a very characteristic feature of this tumor. The hobnail cells are found in virtually every case. The intracytoplasmic glycogen, mucinous inclusions and eosinophilic or hyaline cytoplasmic PAS positive globules can be demonstrated. On immunohistochemistry, these are positive for AE1/3, CK7, and EMA, CA-125 and negative for CD10, inhibin and PLAP. Despite their aggressive clinical course, they are treated like epithelial carcinomas of ovary. The survival rates of patients with advanced clear cell ovarian carcinoma are lower than those of patients with advanced serous type ovarian cancers. The poor response rate to platinum-based regimens may be related to the intrinsic chemoresistance of these tumors [6].
Other tumors which can be confused with metastatic clear cell ovarian tumor include steroid cell tumors, NOS and dysgerminoma. The steroid cell tumors, NOS present in peri- to postmenopausal females with androgenic or estrogenic symptoms. Microscopically lipid rich tumor cells having clear intracytoplasmic vacuoles arranged in solid sheets, thin cords or columns are seen. They are positive for inhibin and negative for EMA, keratin, CK7, CA125 and PLAP.
Dysgerminomas are composed of tumor nests separated by fibrous septae which are infiltrated with lymphocytes. The tumor cells are large polygonal showing abundant clear glycogen rich cytoplasm and distinct cell membranes. The nuclei are round with vesicular chromatin and prominent nucleoli. These are positive for PLAP, CD117, and OCT 4 and negative for CK7, CK20, EMA, HMWK, CD30 and vimentin.
Metastatic clear cell RCC can be either solid or solid and cystic. Solid component is either uniformly or focally yellow. Microscopically, a relatively uniform picture with diffuse sheets of clear cells or tubules lined by similar cells containing eosinophilic material or blood is seen. The hobnail cells and conspicuous mucin production are exceptional in RCC unlike primary clear cell carcinoma of ovary. Also, typical sinusoidal vascular framework of RCC is not a feature of primary ovarian clear cell carcinoma. These are immunoreactive for CD10, EMA, RCC Ag; negative for CA125, inhibin, PLAP, CK7 and HMWCK. The prognosis of metastatic clear cell ovarian carcinoma is favorable [7]. The standard therapy for metastatic RCC beyond cytoreductive surgery is currently based on tyrosine kinase inhibitors and mammalian targets of rapamycin inhibitors [8].
In our case, presence of characteristic alveolar pattern, typical sinusoidal vascular framework, PAS positivity with diastase sensitivity in tumor cells, immunoreactivity for CD10 and coexpresion of CK and vimentin similar to that seen in renal tumor; immunonegativity for CK 7 and CA-125, absence of hobnailing and fibrotic stroma supported the diagnosis of clear cell RCC metastatic to the right ovary.
Conclusion
Although rare, metastatic clear cell RCC should be kept in mind as a differential diagnosis of clear cell tumors in ovary. The histomorphological and immunohistochemical features can be helpful to reach the correct diagnosis. Distinction is important for its prognostic and therapeutic implications. Early diagnosis of this rare metastatic tumor is important for prompt treatment and better patient survival.
Authors' Contribution
MS: Analysis and interpretation of results.
DV: Literature search and drafting of the manuscript.
AM: Review and editing of the manuscript.
SW: Literature search and drafting of the manuscript.
ST: Concept and design and editing of the final manuscript.
Conflict of Interests
The authors declare that there are no conflicts of interests.
Ethical Considerations
Written informed consent was obtained from patient for publication of this case report.
Funding
None declared
Acknowledgement
None
References
[1].Toquero L, Aboumarzouk OM, Abbasi Z. Renal cell carcinoma metastasis to the ovary: a case report. Cases J. 2009 Jul 14; 2:7472. doi: 10.4076/1757-1626-2-7472. PMID 19829972[Pubmed]
[2].Miigica-Álvarez M, Vázquez Bulnes V, Jalon Monzón A, Watering Sejas FJ. Methroragies as form of presentation of an ovary metastases from a primary renal cell carcinoma. Actas Urol Esp 2010; 34(7):638–652[Pubmed]
[3].Decoene J, Ameye F, Lerut E, Oyen R, Van Poppel H, Joniau S. Renal cell carcinoma with synchronous metastasis to the calcaneus and metachronous metastases to the ovary and gallbladder. Case Rep Med. 2011;2011:671645. doi: 10.1155/2011/671645. PMID: 22028725
[Pubmed]
[4].Guney S, Guney N, Ozcan D, Sayilgan T, Ozakin E. Ovarian metastasis of a primary renal cell carcinoma: case report and review of literature. Eur J Gynaecol Oncol 2010; 31(3):339-41.[[oubmed]
[5].Kato Y, Numata A, Wada N, Iwata T, Saga Y, Hashimoto H, Kakizaki H. A case report of metastatic renal cell carcinoma to the ovary. Acta Urol Jpn 2006; 52: 923-927.
[Pubmed]
[6].Pectasides D, Pectasides E, Psyrri A, Economopoulos T. Treatment Issues in Clear Cell Carcinoma of the Ovary: A Different Entity? The Oncologist 2006; 11:1089-1094
[Pubmed]
[7].Albrizio M, Fianza AL, Gorone MSP. Bilateral metachronous ovarian metastases from clear cell renal carcinoma: a case report. Cases Journal 2009, 2:7083[Pubmed]
[8].Syrios J, Kechagias G, Tsavaris N. Treatment of patients with metastatic renal cell
[9].Carcinoma undergoing hemodialysis: case report of two patients and short literature review. BMC Nephrology 2013, 14:84.

