Research

Primary Diagnosis Of Hirschsprung Disease – Calretinin Immunohistochemistry In Rectal Suction Biopsies, With Emphasis On Diagnostic Pitfalls
1Kok Hing Lim, 1Wei Keat Wan, 1 Tony Kiat Hon Lim,1,Alwin Hwai Liang Loh, 2, Shireen A Nah 3,Kenneth Tou En Chang
- 1 Department of Pathology, Singapore General Hospital,Outram Road, Singapore 169608
- 2 Department of Pediatric Surgery, KK Women’s and Children’s Hospital, 100 Bukit Timah Road, Singapore 229899
- 3 Department of Pathology and Laboratory Medicine, KK Women’s and Children’s Hospital, 100 Bukit Timah Road, Singapore 229899
- Submitted: Monday, October 21, 2013
- Accepted; Tuesday, February 18, 2014
- Published: Saturday, March 15, 2014
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ((http://creativecommons.org/licenses/by/3.0)which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Calretinin immunohistochemistry is an adjunctive diagnostic technique in the primary diagnosis of Hirschsprung disease (HD). Rectal suction biopsies from non-HD patients show calretinin immunoreactivity of small mucosal nerves. Those from HD-patients show absent immunolabeling of these nerves.
Study Design and Materials and Methods
This is a prospective study of rectal suction biopsies taken from 99 patients necessitating investigation for HD in our institution during which calretinin immunohistochemistry was routinely performed. At the end of the study period, all calretinin-stained sections were also subjected to a blinded review by 3 external reviewers.
Results
Of the 27 patients with HD, two false negative results were obtained: one related to technical overstaining and the other to punctate immunoreactivity of deep submucosal hypertrophied nerves. Of the 72 non-HD patients, three false positive results were obtained, all relating to diminished immunoreactivity of previously frozen biopsy specimens. In terms of the blinded slide review, 2 reviewers correctly reported 100 out of 101 biopsies (from 99 patients) while 1 reviewer correctly reported 99 out of 101 biopsies. All discordant findings by the reviewers were the result of examining the sections at low (x40 or x100) magnification only and misinterpreting positive calretinin staining as being absent.
Conclusion
Calretinin immunostaining is a reliable ancillary technique
in the investigation of HD, if its potential pitfalls (described both by
ourselves and previously published studies on this topic) are noted. On this
basis, we attempt to formulate a protocol incorporating routine calretinin
immunohistochemistry for the diagnosis or exclusion of HD.
Keywords
Hirschsprung disease, aganglionosis, calretinin immunohistochemistry
Introduction
Hirschsprung disease (HD) is a congenital disorder of intestinal motility characterized by the absence of ganglion cells in the myenteric and submucosal plexuses of the distal intestine. This condition usually manifests shortly after birth with features of intestinal obstruction (constipation and abdominal distention) and feeding intolerance [1]. Delayed passage of meconium beyond the first 24 hours of life is characteristic, but may not present in 10% of patients. A number also present with chronic constipation in later childhood and even adulthood [2]. A number of hypotheses exist as to the underlying pathology of HD. The most prevalent theory is that it is the result of a failure of normal migration of vagal neural crest cells to form the myenteric and submucosal plexuses of the gut during embryonic development [3], a process which occurs in a cranio-caudal fashion, with ganglionation of the distal rectum occurring last. Another theory is that the ganglion cells arrive at their destination but fail to survive or proliferate [4]. In any case, a variable length of intestine may be affected, with implications on the clinical presentation.
The mainstay of treatment is surgical resection of the aganglionic segment with pull-through of normally ganglionated bowel to the anus, while maintaining normal sphincter function. Accurate and prompt diagnosis of HD is crucial – this allows early institution of preoperative measures such as rectal irrigation for bowel decompression while waiting for definitive surgery, thus lowering the risk of life-threatening Hirschsprung-associated enterocolitis [1,2].The diagnosis of HD relies on the histological evaluation of rectal suction biopsies that sample the rectum at a suitable distance (2 to 3 cm) proximal to the physiologically hypoganglionic zone of the anorectal junction. The premise of a histological diagnosis rests on the fact that in HD, submucosal ganglion cells are not identified and hypertrophic submucosal nerve fibres are usually seen (except in long segment disease) [5,6] in an adequately-sized biopsy containing a sufficiently deep amount of submucosa. Since the critical diagnostic feature is an absence of ganglion cells, specimen adequacy is critical. Quantitative criteria for adequacy of the biopsy have been described, and most authorities and standard texts on paediatric surgical pathology advocate biopsies that are at least 3 mm in size, containing submucosa of at least equal thickness as the overlying mucosa, for which multiple levels (100 in some cases) are examined [7]. The most common method used to obtain biopsy specimens is the suction rectal biopsy technique, which minimizes complications of bleeding and perforation with no loss of adequacy compared to the traditional punch biopsy [8]. This is the method routinely used at our institution (KK Women’s and Children’s Hospital, Singapore). When more tissue or deeper levels are required, the open full thickness rectal biopsy may be performed.
Definitive treatment, i.e. surgical resection, rests upon a firm histopathologial diagnosis of HD which in turn rests upon the absence of ganglion cells in the biopsy. Since absence of a particular histological feature may be a consequence of specimen unrepresentativeness or inadequacy, a suitable ancillary technique will be valuable in providing additional diagnostic certainty. Acetylcholinesterase (AChE) histochemistry has been a widely used ancillary technique since the 1970s [9]. However, this technique has limitations such as the necessity of frozen section processing, interpretative difficulties, equivocal/false positive results, and technical challenges [10,11].With the advent of immunohistochemistry, various immunohistochemical stains have been studied in recent years [12]. Amongst these markers, calretinin has been shown to have the most potential for use as a robust ancillary test. The study by Barshack [13] was the first to demonstrate differences in immunohistochemical staining for calretinin between ganglionic and aganglionic portions of bowel in HD, and subsequent studies by Guinard-Samuel [14], Kapur [15], Holland [16]and Kaçar [17] have lent further support to calretinin as a good surrogate marker for HD.While use of calretinin immunohistochemistry in evaluation for HD appears to be increasing, the published peer-reviewed scientific literature of this technique remains relatively sparse, and a recent standard textbook of paediatric pathology [18] comments that this technique may indeed represent a valuable diagnostic adjunct if the utility of (the calretinin) staining pattern can be confirmed by other authors.It is in the light of these circumstances that we have undertaken this study to document the use of calretinin in the evaluation of rectal suction biopsies in neonates, infants and older children with clinical features necessitating investigation for HD. In addition, this study aims to document potential pitfalls in the evaluation of calretinin immunohistochemistry emerging from our experience with this technique.
Materials and Methods
This is a retrospective study of rectal suction biopsies performed on patients with constipation and other clinical features necessitating investigation for HD over a 4-year period (July 2007 to June 2011) in our institution during which calretinin immunohistochemistry was routinely performed.
Light Microscopic Evaluation of Biopsies
During the period of evaluation, rectal suction biopsies from paediatric patients with features of intestinal obstruction were processed according to a standardised protocol. Biopsies were considered adequate if they measured at least 3 mm in greatest dimension and if they contained submucosa of at least equal thickness as mucosa. An initial 12 haematoxylin and eosin-stained sections on two glass slides and a calretinin-stained section were obtained for every biopsy. If ganglion cells were identified by ordinary light microscopy, the pathology reports were signed out accordingly. If ganglion cells were not identified in the initial 12 sections, additional step sections were obtained to a total of at least 100 sections per biopsy or to paraffin block exhaustion. The biopsies were reported as being supportive of HD at the levels of the biopsies if they met adequacy criteria and if they did not contain ganglion cells in at least 100 levels examined. Note was made of the presence or absence of hypertrophied submucosal nerves, defined as nerves with diameters of at least 40 micrometers.
Calretinin Immunohistochemistry Findings
Calretinin immunohistochemistry was performed using an automated platform (BenchMark ULTRA, Ventana Medical Systems Inc., USA). Sections were pre-treated for 36 minutes with cell conditioner 1 before the addition of a primary mouse monoclonal antibody (Novocastra, Leica Microsystems Ltd, United Kingdom) with a dilution of 1:100 and an incubation time of 32 minutes. 3,3’-diaminobenzidine (DAB) was used as a chromogen and slides were counterstained with haematoxylin. Negative controls were performed with omission of the primary antibody. Immunoreactive mesothelial cells from a section of omental tissue served as the external positive control.
Calretin inimmunohistochemical features were evaluated as follows. As previously described, confluent immunoreactivity of small nerve fibres in the lamina propria and muscularis mucosa was designated as indicative of normally ganglionated bowel. Sections lacking such confluent immunoreactivity were designated as supportive of HD. The presence of morphologically unequivocal ganglion cells served as the gold standard for exclusion of a diagnosis of HD at the level of the biopsy. A diagnosis of HD on a subsequent pull-through resection specimen served as the gold standard for suction biopsy-diagnosed HD. These features were tabulated by two authors (KHL and KTEC) following review of all available slides from each patient.
Blinded Calretinin-Stained Slide Review
Further to this, the calretinin-stained sections of all rectal suction biopsies that formed our study cases were reviewed in a blinded fashion without reference to the corresponding H&E-stained sections by three external reviewers (AHLL, WKW and TKHL). None of these three reviewers are paediatric pathologists (all are general histopathologists; AHLL has an interest in renal and urological pathology, and WKW and TKHL have interests in gastrointestinal and liver pathology). None of the three reviewers have experience in assessing calretinin immunohistochemistry in the context of HD diagnosis. Prior to their evaluation of thetest cases, the three reviewers were provided with teaching cases comprising one case each demonstrating the calretinin staining patterns of normally ganglionated bowel and aganlionic HD bowel. The three reviewers were then asked to indicate their assessment for each calretinin-stained section as “HD”, “not HD” or “indeterminate”. For the indeterminate category, they were asked to indicate the reason.
Results
A total of 105 sets of rectal suction biopsies from 102 patients were evaluated by our department for HSCR over the study period, with repeat biopsies obtained from 3 patients.40 patients were female and 62 patients were male. The age range was two days to 16 years, with 49 neonates (1-28 days), 30 infants (1-12 months) and 23 older children (>12 months). The vast majority of the younger patients presented with failure to pass meconium and features of intestinal obstruction. The older patients generally presented with chronic constipation and suboptimal growth and feeding. 4 sets of biopsies (from 3 patients) did not meet adequacy criteria (from 3 patients) did not meet adequacy criteria, leaving the remaining 101 biopsies (from 99 patients) as our study cases.
Retrospective Review of Biopsies from Patients with HD
There were a total of 29 sets of biopsies from 27 patients. The biopsies from all 27 patients did not contain ganglion cells. Those from 25 patients showed a pattern of negative calretinin immunoreactivity typical of HD. Biopsies from 2 patients (patients A and B) had some features that were potential diagnostic pitfalls.
The biopsies from patient A showed a punctate pattern of calretinin immunoreactivity in a few hypertrophied nerves deep within the submucosa, and the pathologist was at the time of initial reporting unable to commit to a definite diagnosis (figure 1). A repeat biopsy showed similar findings, and in view of the consistent absence of ganglion cells in an adequately sized biopsy, a diagnosis of HD was made thence and later confirmed in the subsequent pull-through resection.
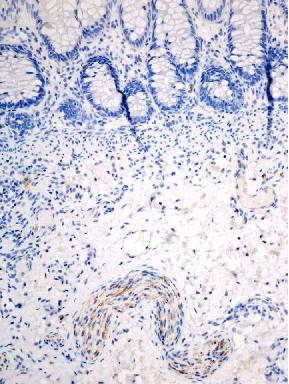
Figure 1: biopsy of rectum with HD. There is punctate (rather than confluent) immunoreactivity in a deep submucosal hypertrophied nerve.
The biopsies from patient B lacked ganglion cells, but showed an apparent reactivity of calretinin of lamina proprial nerve fibres. However, there was in addition marked calretinin overstaining of other cellular structures in the same tissue section. A repeat calretinin stain performed with scrupulous technical attention yielded a typical pattern of negative staining characteristic of HD.
Of these 27 patients with biopsies lacking ganglion cells, a total of 20 (including patients A and B) underwent pull-through endorectal resection of the aganlionic segments, and HD was confirmed in all 20 cases. Of the 7 patients without subsequent resections, 6 were followed up in other institutions, and one died of necrotizing enterocolitis prior to plan resection.
Retrospective Review of Biopsies from Patients without HD
There were a total of 72 sets of biopsies from 72 patients containing ganglion cells. All 72 biopsies showed a pattern of calretinin immunoreactivity featuring confluent staining of small lamina proprial nerve fibres (figure 2). It was further noted that submucosal ganglion cells were calretinin immunoreactive (figure 2) In three patients (patients C1, C2 and C3), frozen section was requested by the surgeon to evaluate for ganglion cells at the time of biopsy. For these three patients, at least two pieces of rectal tissue were obtained, only one of which was submitted for frozen section evaluation. Ganglion cells were identified in all three specimens at the time of frozen section evaluation, and all three were subsequently reprocessed routinely and reassessed as paraffin-embedded sections. It was noted that the calretinin-stained sections of the previously frozen material showed markedly diminished calretinin immunoreactivity of the lamina proprial nerve fibres in comparison to the non-frozen sample from the same patients.
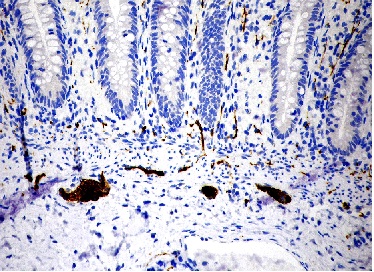
Figure 2 Calretinin immunohistochemistry in a biopsy of normally ganglionated rectum. There is confluent immunoreactivity in mucosal and submucosal nerve fibres, and in ganglion cells.
Blinded Review of Calretinin-stained Sections
Blinded review of only the calretinin-stained sections of the 101 sets of biopsies that formed our study cases by the three external reviewers showed the following results. Two reviewers reported 100 out of 101 biopsies correctly, and one reviewer reported 99 out of 101 correctly. The four discordant diagnoses were four cases of normally ganglionated bowel in which calretinin staining that was present in the small superficial lamina proprial nerves was misinterpreted as being absent. These cases were subsequently reviewed with the external reviewers. In all four cases, the external reviewers came to an agreement that the confluently calretinin-immunoreactive lamina proprial nerve fibres were present with review of the slides under x200 or x400 magnification, and the initial misinterpretations were attributed to overlooking the presence of these small nerve fibres under low (x40 or x100) magnification.
Discussion
Calretinin is a calcium-binding neuronal protein that has been identified in enteric neurones that have projections into mucosal and submucosal layers of the gut [19,20]. The hypothesis that this protein could be used as a marker for aganglionosisin HD has been demonstrated in several studies (table 1). Barshack studied ten large intestinal resection specimens affected by HD, and found that calretinin was not expressed in aganglionic segments whereas ganglionic regions showed immunopositivity of ganglion cells and nerve fibres [13]. Kapur compared the patterns of calretinin immunoreactivity with acetylcholinesterase histochemistry in 31 cases comprising 14 HD and 17 non-HD cases, and concluded that calretinin immunohistochemistry was a potentially superior alternative to acetylcholinesterase staining as an adjunctive diagnostic method for evaluating suction rectal biopsies for HD [21]. Guinard-Samuel reviewed 131 rectal biopsies performed for suspicion of HD comprising 54 HD and 77 non-HD cases; there was good correlation of calretinin immunoreactivity with the presence of ganglion cells and calretin inimmunonegativity with HD [14]. They identified a potential pitfall of ‘slight positive staining of some nerve fibers in HD’, and suggested that this could indicate the beginning of a transitional zone between aganglionic and ganglionic bowel. Holland evaluated 46 rectal suction biopsies from 23 HD and 23 non-HD patients. 4 HD cases were initially deemed negative for intrinsic nerve fibres, but on repeat evaluation, 3 of the 4 cases had foci of pale granular staining on review, and the fourth case was confirmed to be negative for calretinin staining [16], although no explanation was provided. Kaçar assessed resection and full-thickness biopsy material from 33 patients comprising 10 HD and 23 non-HD patients, and found that calretinin staining correlated with disease status in all but one HD patient who had very rare lamina proprialnerve staining at the distal surgical margin [17]. Our own results further support the robustness of this test as an ancillary diagnostic technique.
Translation of these findings to routine diagnostic pathology practice requires identification of pitfalls that confound interpretation, several of which are identified by the present study (table 1),
| Study |
Study cases |
Calretininimmunohistochemistry result |
| Barshack
(2004)13 |
Barshack
(2004)13
|
Expected result in all cases |
| 5 non-HD cases
(resection specimens)
|
Expected result in all cases |
| Kapur
(2008)15
|
14 HD cases
(biopsy specimens)
|
Expected result in all cases |
| 17 non-HD cases(biopsy specimens) |
Expected result in all cases |
| Guinard-Samuel
(2009)14
|
54 HD cases (biopsy specimens) |
Expected result in all cases as assessed by experienced pathologists;
False negative occurrence:one short segment HD case showed slight calretinin staining of submucosalnerve fibres
|
| 77 non-HD cases
(biopsy specimens)
|
Expected result in all cases as assessed by experienced pathologists |
| Holland
(2011)16
|
23 HD cases
(biopsy and resection specimens
|
Expected result in all cases |
| 23 non-HD cases
(biopsy and resection specimens)
|
Expected result in 22 cases;
False positive occurrence: one non-HD case showed absence of lamina proprialnerve fibre staining
|
| Kaçar
(2012)17
|
10 HD cases
(resection specimens)
|
Expected result in 9 cases;
False negative occurrence: one HD case showed lamina proprial nerve fibre staining
|
| 23 non-HD cases
(resection specimens)
|
Expected result in all cases |
| Chang
(2013)
|
27 HD cases |
Expected result in 25 cases
False negative occurrences:
(i) punctate immunoreactivity of deep submucosal hypertrophied nerves;
(ii) technical shortcoming of non-specific immunostaining (‘overstaining’)
|
| 72 non-HD cases |
Expected result in 69 cases
False positive occurrences (same reason in all three cases): diminished calretininimmunoreactivity in a previously frozen biopsy specimen
|
Although fairly obvious, mast cells are calretinin-immunoreactive and should not be mistaken for immunopositive nerve fibres of normally ganglionated bowel. Mast cells demonstrate a fine granular cytoplasmic staining pattern (figure 3) which should not be confused with the dense confluent staining pattern of fine lamina proprial nerve fibres. The presence of mast cells in a biopsy of aganglionic bowel is actually advantageous in that it serves as an internal positive control, providing reassurance that the calretinin stain is technically satisfactory.
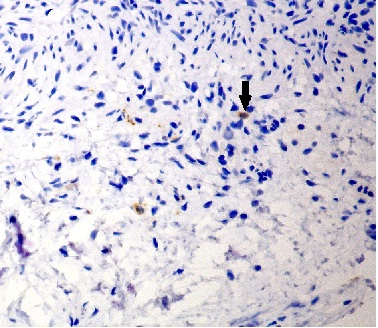
Figure 3 Calretinin immunohistochemistry in a biopsy of rectum with HD. Mast cells can be identified by their oval cellular profiles and by granular cytoplasmic reactivity, and should not be mistaken for the confluent immunoreactivity of nerve fibres present in normally ganglionated bowel.
Secondly, the biopsies of patient A demonstrated punctate (rather than confluent) staining of thick submucosal nerve fibres in a setting of HD. This has been documented previously (Kapur and Guinard-Samuel) and can be a potential source of misinterpretation if situated close to the muscularis mucosae and if it is felt to be substantial enough to approximate the confluent staining of lamina proprial fibres of normally ganglionated bowel.
Thirdly, previously frozen tissue shows a reduction or even complete loss of calretinin immunoreactivity. Calretinin staining and interpretation should therefore be performed on formalin-fixed tissue which has not been previously frozen.
A pitfall not encountered in our series of biopsies but possibly explaining the false negative result of calretinin-immunoreactive nerve fibres in a HD case in the series of cases by Kaçar, is that calretinin-immunoreactive lamina proprial fibres may extend distally from the distal-most portion of ganglionated bowel to the unganglionated segment from which the biopsy is taken. In this situation, although ganglion cells will be absent in the biopsy, interpretation may be confounded by immunoreactive lamina proprial nerve fibres originating from ganglion cells in the adjacent proximal ganglionated segment. This may be a real rather than theoretical pitfall and will mandate continued evaluation of sufficiently sampled biopsies with adequate submucosa.The scenario whereby HD is excluded on the basis of a mucosa-only biopsy sample showing immunoreactive lamina proprial nerve fibres is therefore less likely.
At present, calretinin immunohistochemistry is routinely employed for all rectal suction biopsies obtained for the possibility of HD. The protocol in our institution is as follows. In neonates, surgeons obtain at least 2, sometimes 3 or more rectal suction biopsies from varying distances (typically 2 cm and 2.5 cm) proximal to the dentate line. The specimens are submitted without fixative to allow orientation, but frozen section evaluation is not performed. The specimens are immersed in formalin promptly following orientation. A total of 12 initial levels are examined for the presence of ganglion cells. While calretinin immunohistochemical staining is strictly speaking not required if ganglion cells are present, this is performed routinely to allow familiarity with staining characteristics since this is a fairly new ancillary technique. If ganglion cells are identified, the biopsy is deemed adequate whatever its size. Otherwise, adequacy is determined, for which a size of at least 3 mm in greatest dimension and submucosal thickness at least equal to mucosal thickness are requirements. When ganglion cells are not identified, additional levels are requested for. Typically, a total of at least 100 levels are obtained and examined. This number of levels can usually be laid out on not more than five glass slides if the paraffin blocks are trimmed to a smaller size, facilitating 2 rows of 10 closely spaced levels on a single glass slide. Absence of calretinin immunoreactivity in lamina proprial nerve fibres in this situation is utilised as a supportive finding for a diagnosis of HD.
The situation arises occasionally when a biopsy does not meet adequacy requirements and ganglion cells are not identified. The calretinin stain provides useful information in this situation. If there are immunoreactive lamina proprial nerve fibres, this finding is reported with the comment that this finding is usually seen in normally ganglionated bowel. The surgeon may elect to follow up the patient, and resolution of symptoms on follow up may obviate the need for a re-biopsy. If no immunoreactive lamina proprial nerve fibres are identified, this finding is likewise reported with the comment that this finding usually indicates HD. However, until such time as the evidence base is conclusive, repeat biopsy is advocated to confirm absence of ganglion cells in accordance with past practice.
In conclusion, our experience with calretinin immunohistochemical staining in the evaluation of rectal suction biopsies of children in whom HD is a diagnostic consideration shows that this is a reliable ancillary technique that provides additional diagnostic support for the reporting pathologist if the described pitfalls are noted
(summarized in table 2). We encourage continued reporting of experiences with this ancillary technique so that a stronger evidence base may be built up for the use of this technique in HD diagnosis.
| Causes of false positive results (i.e. no lamina proprial nerve immunoreactivity in non-HD cases): |
| Previous freezing (e.g. for frozen section) of biopsy specimen. |
| Technical shortcoming – failure of staining. |
| Causes of false negative results (i.e. lamina proprial nerve immunoreactivity in HD cases): |
| Biopsy site close to ganglionated segment (as may occur in short segment disease), in which immunoreactive nerve fibres originate from ganglion cells in adjacent proximal ganglionated segment. |
| Misinterpretation of punctate immunoreactivity of deep submucosal hypertrophied nerves (which may occur in HD) as positive immunoreactivity. |
| Misinterpretation of mast cell immunoreactivity as positive immunoreactivity.
Technical shortcoming – non-specific staining (“overstaining”)
|
Authors’ Contributions
KHL reviewed the cases and organized the blinded review, and wrote the draft manuscript.
WKW, TKHL and AHLL participated in the blinded review and reviewed the manuscript.
SAN provided clinical details of the study cohort and reviewed the manuscript.
KTEC conceived the study and study design, oversaw all aspects of the study, and edited the final manuscript. All authors read and approved the final manuscript for publication.
Conflict of Interest
The authors declare that there are no conflicts of interests.
Ethical Consideration
he study was approved by the institutional ethics committee.
Funding
None Declared
Acknowledgements
None
References
[1].Knowles, C.H. et al. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut59, 882-7 (2010).[pubmed]
[2]Coran, A.G., Adzick, N.S., Krummel, T.M., Shamberger, R.C. &Caldamone, A.A. Pediatric Surgery, Seventh Edition.(Saunders, Elsevier, Philadelphia, 2012).
[3]Gershon, M.D. Genes and lineages in the formation of the enteric nervous system. Curr Opin Neurobiol7, 101-9 (1997).[pubmed]
[4]Uesaka, T.&Enomoto, H.Neural precursor death is central to the pathogenesis of intestinal aganglionosis in Ret hypomorphic mice.J Neurosci30, 5211-8 (2010).[pubmed]
[5]Qualman, S.J. & Murray, R. Aganglionosis and related disorders. Hum Pathol25, 1141-9 (1994).[pubmed]
[6].Monforte-Munoz, H., Gonzalez-Gomez, I., Rowland, J.M. & Landing, B.H. Increased submucosal nerve trunk caliber in aganglionosis: a "positive" and objective finding in suction biopsies and segmental resections in Hirschsprung's disease. Arch Pathol Lab Med122, 721-5 (1998).
[7].Qualman, S.J., Jaffe, R., Bove, K.E.&Monforte-Munoz, H. Diagnosis of Hirschsprung disease using the rectal biopsy: multi-institutional survey. PediatrDevPathol2, 588-96 (1999).[pubmed]
[8]Hall, N.J., Kufeji, D. &Keshtgar, A.Out with the old and in with the new: a comparison of rectal suction biopsies with traditional and modern biopsy forceps.J PediatriSurg44(2), 395-8 (2009).[pubmed]
[9]..Meier-Ruge, W. et al. Acetylcholinesterase activity in suction biopsies of the rectum in the diagnosis of Hirschsprung's disease. J Pediatr Surg7, 11-7 (1972).[pubmed]
[10]Pacheco, M.C. & Bove, K.E. Variability of acetylcholinesterase hyperinnervation patterns in distal rectal suction biopsy specimens in Hirschsprung disease. Pediatr Dev Pathol11, 274-82 (2008)[pubmed]
[11].Sun, C.C., Caniano, D.A. & Hill, J.L. Intestinal aganglionosis: a histologic and acetylcholinesterase histochemical study. Pediatr Pathol7, 421-35 (1987).[pubmed]
[12].Kapur, R.P. Can we stop looking? Immunohistochemistry and the diagnosis of Hirschsprung disease. Am J Clin Pathol126, 9-12 (2006).[pubmed]
[13]Barshack, I., Fridman, E., Goldberg, I., Chowers, Y. &Kopolovic, J. The loss of calretinin expression indicates aganglionosis in Hirschsprung's disease. J Clin Pathol57, 712-6 (2004)[pubmed]
[14]Guinard-Samuel, V. et al. Calretinin immunohistochemistry: a simple and efficient tool to diagnose Hirschsprung disease. Mod Pathol22, 1379-84 (2009)[pubmed]
[15]Kapur, R.P. et al. Calretinin immunohistochemistry versus acetylcholinesterase histochemistry in the evaluation of suction rectal biopsies for Hirschsprung disease. Pediatr Dev Pathol, 1 (2008).
[16][Holland, S.K., Ramalingam, P., Podolsky, R.H., Reid-Nicholson, M.D. & Lee, J.R. Calretinin immunostaining as an adjunct in the diagnosis of Hirschsprung disease. Ann Diagn Pathol15, 323-8 (2011)[pubmed]
[17]Kaçar, A., Arikok, A.T., Azili, M.N., Ekberli Agirbas, G. & Tiryaki, T. Calretinin immunohistochemistry in Hirschsprung's disease: An adjunct to formalin-based diagnosis. Turk J Gastroenterol23, 226-33 (2012)[pubmed]
[18].Stocker, J.T., Dehner, L.P. & Husain, A.N. Stocker & Dehner's pediatric pathology, (Lippincott Williams &Wilkins, Philadelphia, 2011).
[19].Rogers, J.H. Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol105, 1343-53 (1987)[pubmed]
[20].Brookes, S.J., Steele, P.A.& Costa, M. Calretinin immunoreactivity in cholinergic motor neurones, interneurones and vasomotor neurones in the guinea-pig small intestine. Cell Tissue Res263, 471-81 (1991).[pubmed]
[21].Kapur, R.P. et al. Calretinin immunohistochemistry versus acetylcholinesterase histochemistry in the evaluation of suction rectal biopsies for Hirschsprung Disease. Pediatr Dev Pathol12, 6-15 (2009). [pubmed]

