Case Report

Encysted variant of papillary carcinoma breast associated with DCIS – a rare case report
*Nagarekha Kulkarni
- *Department of Pathology, Vijayanagara Institute of Medical Sciences, Bellary - 583103. Karnataka, INDIA.
- Submitted: Friday, May 6, 2016
- Accepted: Friday, August 26, 2016
- Published: Saturday, August 27, 2016
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Background
Encysted variant of papillary carcinoma (EPC) is a rare malignancy accounting for 0.5% - 1% of all breast cancers. It is more frequent in elderly postmenopausal women. Patient presents with a lump in the breast, with or without bloody nipple discharge.
Case Presentation
A 60 yrs old female presented with lump in the breast. USG and FNAC findings were inconclusive. So, surgical excision of the cyst along with that surrounding tissue was removed. Histopathologically, a diagnosis of encysted papillary carcinoma of the breast associated with DCIS was made.
Conclusions
The residual palpable mass after a bloody aspirate at FNA is a strong indicator of carcinoma in cystic lesions of the breast.
Key Words
encysted, ductal carcinoma, invasive carcinoma
Introduction
Intracystic papillary carcinomas (IPC) of the breast have traditionally been considered to be variants of ductal carcinoma in situ (DCIS). Later on Carter
et al.,
[1] classified papillary carcinoma of the breast into invasive and non-invasive. With the latter being divided into diffuse and localized form. The diffuse form is the papillary variant of DCIS and the localized form is encysted (intracystic) papillary carcinoma (EPC) which is solitary tumor in a cystic and dilated duct. There are three main subtypes of EPC – pure form, concurrent with DCIS, with concurrent invasion [1]. EPC is a rare malignancy accounting for 0.5% - 1% of all breast cancers. It is more frequent in elderly and more frequent in women than in men [2]. Physical examination and imaging findings are not helpful to diagnose EPC. Fine needle aspiration cytology (FNAC) is inconclusive. Excisional biopsy is usually necessary [3]. Here, with I am reporting a case of EPC associated with DCIS.
Case Presentation
A 60 years old female presented to the surgery out patient with a lump in the left breast since 2 years. Patient was apparently alright 2 years back. Then she noticed lump in the left breast, which was initially small in size and progressed gradually to attain the size of the lemon. There was no past history of fever, loss of weight, cough, breathlessness, hemoptysis and loss of appetite. Family history was insignificant. On palpation, the lump was located in central quadrant of the left breast, hard in consistency, non-tender, no local rise of temperature, mobility was restricted. No
peau de orange was seen. No bloody discharge was present. On general physical examination – there were no palpable lymph nodes, except left axillary lymph nodes which were very small, palpable and were two in number. Cardiovascular system, respiratory system and per abdominal examination were normal. Vital signs were normal. The laboratory tests revealed normocytic normochromic anemia with increase in the blood sugar levels. Ultrasound of the breast revealed loculated abscess on the left central quadrant of breast containing 70 ml of fluid. Rest of the breast parenchyma appears normal. Patient was subjected for fine needle aspiration cytology (FNAC). Aspiration yielded 10 ml of blood mixed fluid material. Five ml was sent for fluid analysis and the findings showed protein – 3.8 g/dl, Sugar – 314 mg/dl; Gram staining – occasional gram negative bacteria; AFB – negative; Cell count – 3200 cells/cmm; Cell tye – polymorphs – 78%, lymphocytes – 22%; RBC – 0.71 million/cmm, Malignant cells were not present. Culture showed no growth. Other 5 ml of fluid was centrifuged and the smears were made from the sediment, fixed and stained with
May-Grünwald Giemsa (MGG) staining and haematoxylin and eosin (H&E) stain. Smears showed cyst macrophages as tiny syncytial aggregates with scattered singly in a blood mixed proteinaceous background. Cytodiagnosis of cystic lesion of breast was made. After a course of antibiotic, under general anaesthesia the lump was excised, along with that a small surrounding tissue was removed and sent for histopathological examination.
Two specimens were received in the histopathology section of the pathology department. Macroscopically the first specimen consists of cystic mass of tissue measuring 5.5 x 4 x 2 cms. Cut section showed a cystic cavity measuring 4.5 x 4 cms filled with hemorrhagic fluid. At one end of the cyst there was grey white solid area measuring 1.5 cms in diameter as shown in (Figure 1) The second specimen was irregular grey white fibrofatty mass of tissue measuring 4 x 2 x 0.5 cms. Cut section showed grey white homogenous areas.
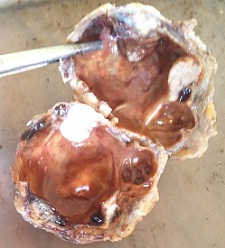
Figure 1: Cut section showed a cystic cavity measuring 4.5 x 4 cm. At one end of the cyst there was grey white solid area measuring 1.5 cm in diameter
Microscopically, sections from the grey white solid areas of the first specimen shows capsulated tumor consisting of neoplastic cells arranged in cribriform, micropapillary pattern which are tightly arranged. This micropapillary pattern shows a well defined fibrovascular core lined by hyperplastic tall columnar epithelium at places showing loss of myoepithelial layer. The nuclei is basally placed, oval and pale to hyperchromatic as shown in (Figure 2).
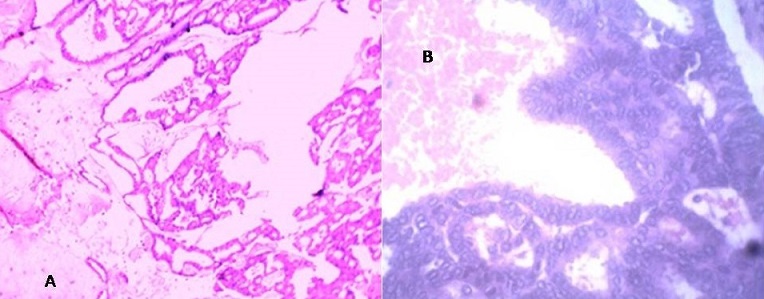
Figure 2: Shows capsulated tumor consisting of neoplastic cells arranged in cribriform, micropapillary pattern which are tightly arranged A. H&E stain 10X; B) H&E stain under 40X
Just below the capsulated area, few neoplastic cells arranged in glandular pattern are seen to be entrapped within the fibrous tissue. Cyst wall shows haemosiderin laden macrophages with secondary giant cell inflammatory reaction. Diagnosis of encysted variant of papillary carcinoma in situ was made. Sections from the second specimen shows neoplastic cells arranged within ducts in a cribriform and micropapillary pattern. Mitosis and necrosis was absent. Diagnosis of duct carcinoma in situ was made. (Figure
3)
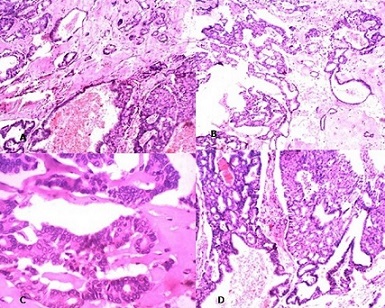
Figure 3: Photomicrigraph showing few neoplastic glands seen in cribriform
pattern outside the capsule - DCIS A) H &E 10X; B) H&E 40X; C) H&E x100X D) H&E
X100
Discussion
EPC is a rare neoplasm of the breast. The overall clinical and radiological presentation is different from DCIS; EPC was made a separate entity. According to Harisrr et al., [4], Leal
et al., [5] and Lefkowitz M et al., [6] nearly 4% to 70% of patients with EPC show DCIS or invasive carcinoma around the tumor. EPC of breast usually occurs in elderly postmenoausal women. In the present case also the age 60 years. There are few cases in the literature of EPC occurring in young women of less than 40 years. Clinical presentation in these patients is painless lump in the breast, with or without bloody nipple discharge. Axillary lymph nodes are infrequent. On ultrasonography the lesions might have an indistinct border of microlobulation, which might suggest malignancy. It is complex in architecture. But in the present study USG findings was loculated abscess collection of left breast. FNAC revealed a cystic lesion. Although ultrasonograhy and FNAC usually the first step in the diagnosis of EPC. Cytological examination also has high false negative results due to necrotic material, degenerative diagnostic cells and obscuring blood in the cystic lesion. Donya Farroh et al., also had the same problem with FNAC [7]. The combination of residual palpable mass and a frankly bloody aspirate at FNA is a strong indicator of carcinoma. Core needle biopsy of the intracystic mass with ultrasonography is a useful tool for diagnosis of EPC, but cannot distinguish between in situ from invasive carcinoma. In the present study surgical excision was done and Tomonori et al also found that surgical excision was necessary because EPC can be further classified. Histopathological, we noticed cribriform and papillary pattern of neoplastic cells and DCIS was seen in the surrounding breast tissue. Identification of myoeithelial cell layers essentially by IHC analysis has become a key feature in distinguishing benign from malignant and in situ from invasive papillary carcinoma of breast.
When the EPC occurs as pure form the prognosis is good. The presence of DCIS or IDC in the surrounding breast tissue is associated with increased risk of local recurrence for DCIS and metastasis for the IDC. In the present EPC was associated with DCIS in the surrounding tissue.
Calderaro et al., [8] found twenty cases in their study confirmed the low malignancy and excellent prognosis of EPCs. Seal
et al., [9] in their study of five cases of EPC consider this tumor as uncertain malignant potential which should not be treated like invasive ducatal carcinoma of the breast.
Wynveen et al., proposes that along with surgery adjuvant radiotherapy and hormone therapy should be given, because these EPC is positive for oestrogen for receptors but negative for HER2. They also propose sentinel lymph node biopsy as a surgical option even if axillary lymph node metastases of EPC are extremely rare [10].
Conclusions
Encysted papillary carcinoma associated with DCIS is a rare malignancy. More common in the elderly women. FNAC has high false negative results. The combination of residual palpable mass and a frankly bloody aspirate at FNA is a strong indicator of carcinoma. Surgical excision of the cyst along with sentinel lymph node biopsy is the treatment of choice.
Author contribution
NK conceived and designed the study did the literature search and prepared the manuscript.
Conflict of Interests
The author declares that there is no conflict of interest.
Ethical considerations
The written informed consent was taken from the patient for publication of this case report. A copy of the report is available with the author.
Funding
None
References
[1].Carter D, Orr SL, Merino MJ. Intracystic papillary carcinoma of the breast. After mastectomy, radiotherapy or excisional biopsy alone. Cancer. 1983 Jul 1; 52(1):14-9 [PubMed]
[2].Rosen PP. Papillary carcinoma. In: Rosen PP. ed. Rosen's Breast Pathology, First edition. Philadelphia, Lippincott-Raven; 1997:335-354.
[3].Solorzano CC1, Middleton LP, Hunt KK, Mirza N, Meric F, Kuerer HM, Ross MI, Ames FC, Feig BW, Pollock RE, Singletary SE, Babiera G. Treatment and outcome of patients with intracystic papillary carcinoma of the breast. Am J Surg. 2002 Oct; 184(4):364-8 [PubMed]
[4].Harris KP, Faliakou EC, Exon DJ, Nasiri N, Sacks NP, Gui GP. Treatment and outcome of intracystic papillary carcinoma of the breast. Br J Surg. 1999 Oct; 86(10):1274 [PubMed]
[5].Leal C, Costa I, Fonseca D, Lopes P, Bento MJ, Lopes C. Intracystic (encysted) papillary carcinoma of the breast: a clinical, pathological, and immunohistochemical study. Hum Pathol. 1998; 29(10):1097–104.
[Pubmed]
[6].Lefkowitz M, Lefkowitz W, Wargotz ES. Intraductal (intracystic) papillary carcinoma of the breast and its variants: a clinicopathological study of 77 cases. Hum Pathol. 1994; 25(8):802–9 [PubMed]
[7].Farrokh D, Abedi M, Fallah Rastegar Y. An intracystic papillary carcinoma of the breast. Iran J Cancer Prev. 2013 Spring;6(2):118-21 [PubMed]
[8].Calderaro J, Espie M, Duclos J, Giachetti S, Wehrer D, Sandid W, Cahen-Doidy L, Albiter M, Janin A, de Roquancourt A. Breast intracystic papillary carcinoma: an update. Breast J. 2009;15(6):639–44.[PubMed]
[9].Seal M, Wilson C, Naus GJ, Chia S, Bainbridge TC, Hayes MM. Encapsulated apocrine papillary carcinoma of the breast--a tumour of uncertain malignant potential: report of five cases. Virchows Arch. 2009 Dec;455(6):477-83. doi: 10.1007/s00428-009-0834-7. Epub 2009 Oct 28.
[Pubmed]
[10].Wynveen CA, Nehhozina T, Akram M,
Hassan M, Norton L, Van Zee KJ, Brogi E. Intracystic papillary carcinoma of the
breast: An in situ or invasive tumor? Results of immunohistochemical analysis
and clinical follow-up. Am J Surg Pathol. 2011 Jan;35(1):1-14. doi:
10.1097/PAS.0b013e3181fbe20a. [Pubmed]

