Research

EGFR over-expression in Head and Neck Squamous Carcinoma & it's correlation with etiological factors like alcohol and tobacco
1 Vineeta Gaur nee Srivastava, 2 Mohan Kumar 3T.P. Chaturvedi, 1Manoj Pandey*
- 1Department of Surgical Oncology, Institute of
Medical Sciences, Banaras Hindu University, Varanasi 221005, India
- 2Department of Pathology, Institute of Medical
Sciences, Banaras Hindu University, Varanasi 221005, India
- 3Institute of Medical Sciences, Faculty of Dental Sciences
- Submitted: Monday, 20 June 2016
- Accepted: Tuesday, December 27, 2016
- Published: Tuesday, December 27, 2016
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Objectives
The present study was carried out to investigate the possible association of EGFR expression with personal habit as possible risk factors for development of HNSCC.
Study design
Immunohistochemical analysis was performed on the samples obtained from 103 patients with HNSCC, 25 patients with pre-cancerous condition and 116 healthy controls. The data was analyzed using parametric and non parametric tests. The categorical data was analyzed by chi square test. Correlation analysis was also performed.
Results
In our study in carcinoma group, correlation coefficient for personal habit status of the patient with EGFR expression was -0.031 and p=0.755 which is not significant. The EGFR expression intensity is higher in cancer patients compared to the normal subjects. This difference is statistically significant (p=0.000).
Discussion
EGFR expression was high in patients with oral cancer compared to premalignant and malignant diseases. However, our study does not show any association of risk of development of HNSCC due to the consumption of cancer causing products to personal habits of the subjects.
Introduction
Head and neck cancers are considered among the 10 most common cancers globally [1]. According the International Classification of Diseases (ICD), Head and Neck cancer can occur in oral cavity, pharynx and the larynx (ICD-10, C00.0-14.0). Oral cavity cancers include cancers of the tongue, mouth, gum, floor of the mouth, palate and other unspecified parts of the mouth (ICD-10, C01.0-06.0). In developed countries HNSCC accounts for 6% of all cancers whereas in developing countries it accounts for 30% [2]. 90% cases of H&N cancer are squamous cell carcinoma as they arise from the squamous cell epithelial of the mucosal lining [3]. About one fourth of all cancer diagnosed in men is Head and Neck Squamous cell Cacrcinoma (HNSCC) while, in case of females, only one tenth of all the cancers diagnosed are HNSCC [1]. Men are affected twice as often as compared to women. Of all the cancers head and neck cancers in India accounted for 30 % in male and 11-16 % mortality in female. Over 200,000 cases of Head and Neck cancers occur each year and nearly 80,000 are diagnosed every year in India. Cancer of the oral cavity is the major site in India whereas cancer of the pharynx is the most common site in France [4]. In India, Dibrugarh (Assam) has reported the highest incidence of head and neck cancers i.e. 49.6%. [2]. More than 90% of oral cancers occur in patients older than 45 years of age [5].
The overall 5-year-survival rate is still around 50-60% [6]. Despite many advances in the treatment there is no respectable improvement in the survival rate of head and neck squamous cell carcinoma. Patients are presented with advanced stage of the disease [7]. Even after the treatment of current era like surgery, radiation chemotherapy, patients areleft with adverse effect of compromised face, speech and swallowing [8]. This ultimately diminished quality of life of the patients.
Etiologic agents involved in the development of Head and neck cancers include tobacco, alcohol consumption, diet, genetic susceptibility, certain chemicals, and radiations in addition to viral infections such as exposure to human papilloma virus (HPV) [9- 11]. Tobacco and alcohol usage are the major risk factors, which lead to nutritional deficiencies, and susceptibility to various carcinogens and thus lead to immune suppression. Seventy five percent of head and neck cancers are attributed to tobacco and alcohol usage. Heavy alcohol drinkers are frequently heavy smokers as well [12- 15]. The risk for development of oral cancer is 3 to 9 times greater in those who smoke or drink and as much as 100 times greater in those who both smoke and drink heavily than in those who neither smoke nor drink [16]. A wide variety of tobacco habits like bide smoking, tobacco chewing, and cigarette smoking account for a large majority of these cancers [17]. Strategies for prevention of H&N cancer might be much more effective if the individuals with increased risk could be identified before they develop HNSCC.Over expression of growth factor is an added cause for cancer. These growth factors work as intermediate for several signal pathways. In head &neck cancer epidermal growth factor receptor (EGFR) is the most important growth factor that has been studied. A mature EGFR is a 170 kDa transmembrane glycoprotein. It is composed of a single polypeptide chain of 1186 amino acids residues [18- 20].
Proteins dock on phosphorylated residues, leading to the activation of signaling pathways that promote cell growth, proliferation, differentiation, and migration. ErbB family ligands are EGF and transforming growth factor-a (TGF-a). The epidermal growth factor family of receptor tyrosine kinases consists of four receptors, EGFR (ErbB1), ErbB2 (Her2/Neu), ErbB3 (Her 3) and ErbB4 (Her 4). ErbB1 is also known as EGFR and HER. Alteration in the function of EGFR has been linked with oncogenic transformation, autonomous cell growth, invasion, angiogenesis and development of metastasis in several cancers. The present study was carried out to investigate the possible association of EGFR expression with personal habit factors acting as possible risk factors for development of HNSCC. Therefore, we compared the expression of EGFR by immunohistochemistry in normal individual patients with precancerous stage and patients of HNSCC using various parameters.
Material and method
The study was carried out in the department of surgical oncology, IMS, BHU from July 2007- Dec 2013. For sample collection of cancerous and premalignant cases, patients attending the O.P.D and those admitted in the Department of Surgical Oncology, Sir Sunder Lal hospital, BHU were selected. For normal subjects samples were taken from the Faculty of Dental Sciences, Sir Sunder Lal Hospital, BHU, Varanasi. The precancer lesions were classified clinically as described in earlier studies [21- 23].
Sample collection
A total of 103 patients with HNSCC, 116 samples of subjects with normal oral cavity and 25 samples of subjects with precancer lesions were included in the study. A detailed history was taken and examination was carried out after obtaining the written informed consent from all the patients. The study was approved by the Ethical Committee of the Institute of Medical Sciences. Personal habits like use of tobacco and alcohol were recorded and details of their consumption were also recorded.
Immunohistochemical Analysis of EGFR localization
Tissue processing: Tissues obtained from normal individuals and patients fixed in formalin, paraffin blocks prepared and 4 micron sections cut. The sections were then processed for immunohistochemistry as outlined below
Immunohistochemistry for EGFR
4 µm sections were cut from the blocks and were deparaffinized in xylene followed by hydration in a graded series of alcohols. Sections were left under running water for 15 minutes. Endogenous peroxidase activity was blocked by incubation in 3% H2O2 for 30 minutes at room temperature. After rinsing in TBS buffer (Ph7.4, for 30 min.), the sections were incubated with primary antibody against EGFR at 4°C overnight. After 3 washing with tris buffer for 10 min. each, covered the sections with secondary antibody. The details of primary and secondary antibodies used are detailed below. Wash the samples in TBS (3×10’). Incubate the section in ABC solution for 30 min. After 3 TBS washing, sections were counterstained with 3-3’-diaminobenzidine (DAB) followed by hematoxylin. Slides were washed in running water and were mounted with DPX. EGFR SC-03 Monoclonal antibody (Santa Cruz, CA, USA) was used with Vectastain Elite ABC detection system.
The degree of EGFR was assessed quantitatively in each group by image analysis using the following score:IHC score
0 = undetectable levels, IHC score (+) = 10-30 % immunoreactivity,
IHC score (++) = 30-60% immunoreactivity, IHC score (+++) = >60% immunoreactivity
Statistical analysis
The variables in the study were correlated with expression levels of EGFR and other patient data. The data have been analyzed using parametric and non parametric tests. The categorical data has been analyzed by chi square test. Correlation analysis has been done.
Results
In cancer group, 13.6% patients have habit of chewing tobacco, 1% has habit of smoking, 1% is involved in drinking alcohol and 9.7% are using areca nut. 20.4% are involved in all the activities. 2.9% patients are using tobacco for chewing as well as for smoking also. 5.8% patients are taking both, tobacco in the form of chewing and alcohol. Highest percentage of the patients i.e. 22.3% are taking tobacco for chewing in combination with areca nut. 3.9% patients have habit of chewing areca nut simultaneously with smoking. 5.8% patients have habit of chewing tobacco, smoking and also drinking alcohol. 1.9% patients are areca nut chewer with habit of taking tobacco in the form of chewing and smoking both. 8.7% are taking alcohol along with chewing tobacco and areca nut. However there are also 2.9% patients of Head and Cancer who do not have any of these habits (Figure 1)
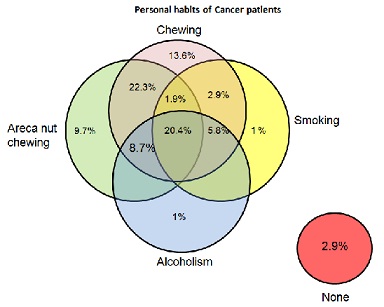
Figure 1: Personal habits of patients of cancer group
(Figure 2) depicts the personal habit of patients in precancerous group. In precancerous group 4% patients are addicted to tobacco chewing, 1% for smoking, 1% for alcohol and 4 % are for areca nut chewing. The number is also higher in patients (28%) who have habits of chewing tobacco and areca nut both. 4% patients are habitual to use tobacco for chewing and smoking, 12% for chewing, smoking and alcohol, 4% for smoking, alcohol and chewing areca nut. 8.7% patients are using alcohol with chewing habit of areca nut. As in cancer group, precancerous patients also have a habit of all chewing tobacco, smoking, drinking alcohol and chewing areca nut and the number of patients with these habits is 28%.
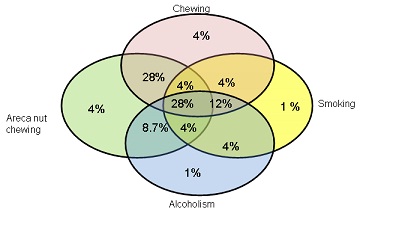
Fig 2: Personal habits of patients of precancerous group:
EGFR expression in all three groups
Patients in all the three groups expressed EGFR. 69/116 of the normal subjects, 24/25 patients with premalignant lesions and 100/103 cancer patients expressed EGFR. However the intensity is higher in cancer patients compare to normal subjects. This difference is statistically significant (p=0.000).(Table 1)
Table 1:
Intesnity of EGFR expression in three groups
|
Intensity of staining
|
Normal
|
Premalignant
|
Malignant
|
|
0
|
47
|
1
|
3
|
|
1
|
66
|
3
|
15
|
|
2
|
3
|
8
|
27
|
|
3
|
0
|
13
|
58
|
Table 2 shows cross tabulation of EGFR expression in Cancer, precancerous and normal groups by IHC with various factors.
Table 2:
EGFR expression analysis by IHC with various factors
|
|
|
Carcinoma
|
Precancerous
|
Normal
|
|
|
|
0
|
1
|
p value
|
0
|
1
|
p value
|
0
|
1
|
p value
|
|
Gender
|
Male
Female
|
3
0
|
89
11
|
.543
|
1
0
|
20
4
|
.656
|
26
21
|
46
23
|
.216
|
|
Total
|
|
|
1
|
24
|
|
47
|
69
|
|
|
Marital statuts
|
Married
Unmarried
|
3
O
|
98
2
|
.805
|
1
0
|
23
1
|
.835
|
-
|
-
|
-
|
|
Total
|
3
|
11
|
|
1
|
24
|
|
|
|
|
|
Religion
|
Hindu
Others
Total
|
3
0
3
|
92
8
10
|
.610
|
0
1
1
|
23
1
24
|
.001
|
-
|
-
|
-
|
|
Family History
H&N
|
Yes
NO
|
0
3
|
2
98
|
.805
|
0
1
|
0
24
|
|
-
|
-
|
-
|
|
Family History
Any Ca
|
Yes
NO
|
0
3
|
10
90
|
.564
|
0
1
|
2
22
|
.763
|
-
|
-
|
-
|
|
Personal Habit
Status
|
Current
Past
Never
|
1
2
0
|
13
84
3
|
.583
|
1
0
0
|
13
11
0
|
.366
|
-
|
-
|
-
|
|
Personal Habit
Amount
|
Daily
Occasional
Never
|
3
0
0
|
92
5
3
|
.878
|
1
0
|
23
1
|
.835
|
-
|
-
|
-
|
EGFR expression is cross tabulated with the marital status of carcinoma and precancerous patients. Out of 101 married patients of carcinoma group, 98 show EGFR expression and only 3 in widowed. p=0.805.In precancerous group, EGFR expression have been shown in 23 married and 1 widowed patient and the p value is 0.835. Marital status of patients has no significant role with EGFR expression by IHC.
In cancer group, 95 Hindu patients have been included. Out of 95, 92 patients are showing EGFR expression and the p value is 0.610.In precancerous group 23 Hindu patients have shown positive results for EGFR by IHC and the p value is 0.001. This p value is mainly because of the higher number of Hindu patients in this group. Out of 25 patients, 23 patients are of Hindu religion.
Out of 103 patients in carcinoma group, only 2 patients have history of Head and Neck cancer in their family and both the patients have EGFR expression by IHC. 98 patients who don’t have a history of Head and Neck cancer in their family express EGFR by IHC. There is no significance (p=0.805) of family history of Head and Neck Cancer with EGFR expression by IHC. In precancerous group, all the patients included in this study don’t have family history of Head and Neck cancer. Except one patient, all have shown EGFR expression. P value can’t be calculated for this factor.
EGFR expression has been cross tabulated with history of any type of cancer in patient’s family. In Cancer group, 10 patients who have history of any type of cancer in their family Express EGFR and 90 patients who do not have history of any type of cancer express EGFR by IHC. pvalue is 0.564. In precancerous group p value is 0.763. Both the patients with family history of any type of cancer in their family have shown EGFR expression and 22 patients who do not have this type of history express EGFR by IHC. p values of both the groups show that there is no significance.
In the cross tabulation of EGFR expression with patients habit status, 84 in carcinoma group who left the habit of chewing or smoking tobacco, alcohol and areca nut have shown positive results for EGFR expression. In precancerous group also the highest number of patients for positive EGFR expression is of patients who have left these habits. p value for cancer group is 0.583 and for precancerous group is 0.366 and both values show that there is no significance of habit status with EGFR expression by IHC.
Majority of the patients who take these carcinogenic products daily express EGFR by IHC in carcinoma group and same results are in precancerous group. p value for cancer group is 0.878 and for precancerous is 0.835. EGFR expression has no significance with this factor.
Discussion
EGFR is a transmembrane tyrosine kinase receptor of the erbB-family that is normally expressed at low levels on the surface of most normal cells. EGFR is over expressed in head and neck tumors. Over expression of EGFR has been associated with a more aggressive clinical behavior, resistance to treatment and a poor prognosis. Studies have shown EGFR over expression as an independent prognostic marker of survival in betel quid chewers and a component of a prognostically significant molecular profile. Signal transduction from activated transmembrane receptors like EGFR depends on a variety of downstream mediators that are frequently altered in various cancer types.
Activation of the EGFR results in the initiation of a diverse array of cellular pathway. In response to toxic environmental stimuli, such as ultraviolet irradiation, or to receptor occupation by EGF, the EGFR forms homo- or heterodimers with other family members [24].
Each dimeric receptor complex initiates a distinct signaling pathway by recruiting different Src homology 2 (SH2) -containing effecter proteins. Dimerization results in autophosphorylation initiating a downstream cascade culminating in cellular responses such as cell proliferation or apoptosis. The activated EGF-R dimer complexes with the adaptor protein, Grb, coupled to the guanine nucleotide releasing factor, SOS. The Grb-SOS complex can either bind directly to phosphotyrosine sites in the receptor or indirectly through Shc. These protein interactions bring SOS in close proximity to ras, allowing for ras activation. This subsequently activates the ERK and JNK signalling pathways that, in turn, activate transcription factors, such as c-fos, AP-1, and Elk-1 that promote gene expression and contribute to cell proliferation [25].
Alcohol and smoking also increase the EGFR expression. The role of EGFR in the development of premalignant tissue changes which are probably influenced by chronic toxic irritation. EGFR2 (Her 2/New) can also be used as a marker in distinguishing normal oral mucosa (NOM)/epithelia l dysplasia (ED) from OSCC. The significant increase of Her 2 makes it a valuable marker for OSCC. Not only EGFR gene’s amplification is responsible for squamous cell carcinoma of the head and neck but also the increased EGF binding capacity [26]. EGFR also indirectly helps in tumor formation. EGFR directly interacts with b- catenin which is an E- cadherin- mediated cell adhesion molecule. This interaction includes tyrosine phosphorylation of b- catenin which causes dysfunction of the E- cadherin- mediated cell adhesion in cancer, resulting in increased cell motility, invasion and metastasis [27].
In our study personal habit of patients have not shown any significance with EGFR expression. In carcinoma group, correlation coefficient for personal habit status of the patient was -0.031 and p=0.755. It has been found that individuals of all the three groups expressed EGFR. However the intensity is higher in cancer patients compare to normal subjects. This difference is statistically significant (p=0.000). Few other studies also showed relationships between the above mentioned etiological factors and development of HNSCC and its prognosis [27- 36]. However, further studies are necessary to clarify the role of these factors in modulating the EGFR expression and its function.
Competing interests
The author(s) declare that they have no competing interests.
Funding
The SRF of Dr. Vineeta Srivastava was supported by Indian
Council of Medical Research
Acknowledgement
None
Ethical Considerations
Authors declare that the present study was approved by the Institute Ethics Committee.
Abbreviations
Head and Neck Squalors cell Carcinoma (HNSCC), epidermal growth factor receptor (EGFR), Head and neck cancer (H&N cancer), Oral Squalors cell carcinoma (OSCC)
References
-
Yeole BB. An Assessment of Improvement in Reliability and Completeness of Mumbai Cancer Registry Data from 1964-1997. Asian Pac J Cancer Prev 2001;2(3):225-32.[Pubmed]
-
National Cnacer Registry Programme (ICMR). Consolidated Report of population Based Cancer Registries: 2004-2005. Bangalore, India: Indian Council of Medical Research; 2008.
-
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005 March;55(2):74-108
[Pubmed]
-
Coleman MP, Esteve J, Damiecki P, Arslan A, Renard H. Trends in Cancer incidence and mortality. IARC; Lyon, France, 1993.[Pubmed]
-
Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973-1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg 2002 March;128(3):268-74
[Pubmed]
-
Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents Vol. VIII. Lyon, France: IARC, 2002.
-
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet 2008 May 17;371(9625):1695-709
[Pubmed]
-
Deleyiannis FW, Weymuller EA, Jr., Coltrera MD. Quality of life of disease-free survivors of advanced (stage III or IV) oropharyngeal cancer. Head Neck 1997 September;19(6):466-73
[Pubmed]
-
Jaiswal R, Pandey M. Human papilloma virus transmission to oral cavity: an unresolved mystery. World Journal of Epidemiology and Cancer Prevention 2012 February 6;1(1).
-
Mankodi RR, Singh R, Jaiswal R et al. A paired group study to evaluate the perinatal transmission of Human papilloma virus in high prevalence area. World Journal of Epidemiology and Cancer Prevention 2012;1(2).
-
Kumar A, Kumar M, Jaiswal R, Shrivastava V, Dixit R, Pandey M. Presence of Human papilloma virus and EGFR expression does not predict response to Neoadjuvant chemotherapy in oral cancer. World Journal of Surgical Medical and Radiation Oncology 2012;1(20).
-
Control of oral cancer in developing countries. A WHO meeting. Bull World Health Organ 1984;62(6):817-30
[Pubmed]
-
Gupta PC. Survey of sociodemographic characteristics of tobacco use among 99,598 individuals in Bombay, India using handheld computers. Tob Control 1996;5(2):114-20.[Pubmed]
-
Gupta PC, Mehta HC. Cohort study of all-cause mortality among tobacco users in Mumbai, India. Bull World Health Organ 2000;78(7):877-83.[Pubmed]
-
Yeole BB. Trends and predictions of cancer incidence cases by site and sex for Mumbai. Indian J Cancer 1999 June;36(2-4):163-78
[Pubmed]
-
Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin 2002 July;52(4):195-215
[Pubmed]
-
Sanghvi LD, Rao DN, Joshi S. Epidemiology of head and neck cancers. Semin Surg Oncol 1989;5(5):305-9.
[Pubmed]
-
Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem 1987;56:881-914.:881-914
[Pubmed]
-
Cohen S, Carpenter G, King L, Jr. Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem 1980 May 25;255(10):4834-42.
-
Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem 1988;57:443-78.:443-78
[Pubmed]
-
Sankaranarayanan R, Mathew B, Jacob BJ et al. Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. The Trivandrum Oral Cancer Screening Study Group. Cancer 2000 February 1;88(3):664-73.
-
Pandey M, Thomas G, Somanathan T et al. Evaluation of surgical excision of non-homogeneous oral leukoplakia in a screening intervention trial, Kerala, India. Oral Oncol 2001 January;37(1):103-9.
-
Ramadas K, Sankaranarayanan R, Jacob BJ et al. Interim results from a cluster randomized controlled oral cancer screening trial in Kerala, India. Oral Oncol 2003 September;39(6):580-8
[Pubmed]
-
Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 1996 November 15;274(5290):1194-7.
-
Qian X, Vass WC, Papageorge AG, Anborgh PH, Lowy DR. N terminus of Sos1 Ras exchange factor: critical roles for the Dbl and pleckstrin homology domains. Mol Cell Biol 1998 February;18(2):771-8
[Pubmed]
-
Rikimaru K, Tadokoro K, Yamamoto T, Enomoto S, Tsuchida N. Gene amplification and overexpression of epidermal growth factor receptor in squamous cell carcinoma of the head and neck. Head Neck 1992 January;14(1):8-13[Pubmed]
-
Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J 2004 April 21;23(8):1739-48.
-
Mayer A, Zahnreich S, Brieger J, Vaupel P, Schmidberger H. Downregulation of EGFR in hypoxic, diffusion-limited areas of squamous cell carcinomas of the head and neck. Br J Cancer 2016 November 22;115(11):1351-8
[Pubmed]
-
Schulz D, Wirth M, Piontek G et al. HNSCC cells resistant to EGFR pathway inhibitors are hypermutated and sensitive to DNA damaging substances. Am J Cancer Res 2016 September 1;6(9):1963-75
[Pubmed]
-
Sun Y, Sang Z, Jiang Q, Ding X, Yu Y. Transcriptomic characterization of differential gene expression in oral squamous cell carcinoma: a meta-analysis of publicly available microarray data sets. Tumour Biol 2016 October 4.[Pubmed]
-
Gao J, Ulekleiv CH, Halstensen TS. Epidermal growth factor (EGF) receptor-ligand based molecular staging predicts prognosis in head and neck squamous cell carcinoma partly due to deregulated EGF- induced amphiregulin expression. J Exp Clin Cancer Res 2016 September 26;35(1):151.
-
Bossi P, Resteghini C, Paielli N, Licitra L, Pilotti S, Perrone F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget 2016 August;10
[Pubmed]
-
Beck TN, Georgopoulos R, Shagisultanova EI et al. EGFR and RB1 as Dual Biomarkers in HPV-Negative Head and Neck Cancer. Mol Cancer Ther 2016 October;15(10):2486-97
[Pubmed]
-
Baschnagel AM, Tonlaar N, Eskandari M et al. Combined CD44, c-MET, and EGFR expression in p16-positive and p16-negative head and neck squamous cell carcinomas. J Oral Pathol Med 2016 July 21;10
[Pubmed]
-
Tepper SR, Zuo Z, Khattri A, Hess J, Seiwert TY. Growth factor expression mediates resistance to EGFR inhibitors in head and neck squamous cell carcinomas. Oral Oncol 2016 May;56:62-70. doi: 10.1016/j.oraloncology.2016.03.008. Epub@2016 Mar 26.:62-70
[Pubmed]
-
Cimpean AM, Balica RA, Doros IC et al. Epidermal Growth Factor Receptor (EGFR) and Keratin 5 (K5): Versatile Keyplayers Defining Prognostic and Therapeutic Sub-classes of Head and Neck Squamous Cell Carcinomas. Cancer Genomics Proteomics 2016 January;13(1):75-81.

