Review

Vertebral metastases from oral squamous cell carcinoma of the buccal mucosa – report of a case and review of literature.
Latha P. Rao1, Seema Singh2, Mridula Shukla3, Manoj Pandey4
- 1Department of Oral and Maxillofacial Surgery, Amrita School of Dentistry, Kochi, India
- 2Departments of Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- 3Department of Pathology, Religere labs, Lanka, Varanasi, India
- 4Departments of Surgical Oncology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- Submitted: May 2, 2012;
- Accepted June 9, 2012,
- Published: June 11, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Oral squamous cell carcinoma (OSCC) is the most common head neck malignancy having a propensity for loco-regional spread. Hematogenous spread is rare with only a few existing reports in the literature. With improved loco-regional control of the primary disease now possible with newer treatment modalities, more and more cases of distant metastasis from OSCC are being detected. Most frequently reported sites of metastasis from OSCC include lung, bone, liver, adrenals, heart, and kidney. Here we present a case of squamous cell carcinoma of the buccal mucosa with metastasis to vertebrae with a brief review of the literature on osseous metastases from OSCC.
Introduction
Squamous cell carcinoma (SCC) is the most common malignant neoplasm of the oral cavity and represents about 90% of all oral malignancies [1]. Oral squamous cell carcinoma (OSCC) has traditionally been considered a loco-regional disease, with distant metastasis from primary site being rare. Distant metastatic spread from OSCC is thought to be by the hematogenous route either from the primary site or from the cervical lymph nodes that have been infiltrated by the oral squamous cell carcinoma. Crile reported an incidence of 1% for the distant metastasis from 4500 cases of head and neck squamous cell carcinomas (HNSCC) reviewed by him [2]. He stated that “the collar of lymphatics about the neck forms an almost impassable barrier through which cancer rarely penetrates” signifying the rarity of hematogenous speread [3]. Subsequent studies have shown the incidence of distant metastases to be much higher. Many investigators have reported the incidence of clinically detected distant metastases in squamous cell cancer of the head and neck, ranging from 11 to 23 percent [4-7]. The prevalence of distant metastases at autopsy (37–57%) is much higher than in clinical studies [4, 8-18].
Most frequent sites of metastasis from cancer of the oral cavity include lung, bone and bone marrow, liver, adrenal, heart, mediastinum, skin, and kidney [4,12,16,19-21]. Osseous involvement has been reported with varying frequencies and almost always second only to pulmonary involvement [22]. Here we present a case of squamous cell carcinoma of buccal mucosa which showed metastases to vertebrae and review the literature on distant bone metastases in OSCC.
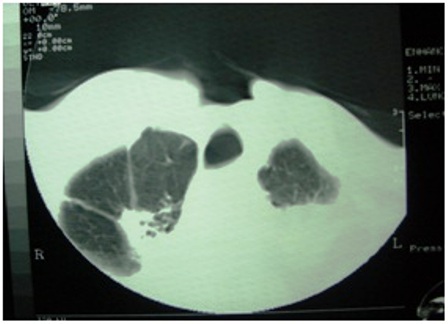
Figure 1: Computerized tomography of the chest showing pulmonary tuberculosis
Case report
A 55 year old male presented with a painful, non-healing ulcer on the left side of cheek noticed 3 months back. He was habituated to chewing pan with betel nut, tobacco and lime and keeping the quid in the buccal sulcus. On clinical examination an 3.5 X 3.0 cm ulceroproliferative lesion was found on the left buccal mucosa, extending from canine to retromolar trigone posteriorly and from lower gingivobuccal sulcus to superior gingivobuccal sulcus. He had clinically palpable lymph nodes of 0.5 X 0.5cm size at level Ib and II. His disease was clinically staged as T3N2a. Patient had trismus and his mouth opening was 15 mm. Histopathology examination of the punch biopsy showed moderately differentiated squamous cell carcinoma (MDSCC). Patient was treated with primary concomitant chemoradiation using methotrexate 50 mg/m2 with BDX external radiation to the dose of 52.5 Gy/15 F. Four weeks after the radiation there was a residual lesion in the primary, a salvage surgery was advised for the residual tumor which was refused by the patient and he was lost to follow-up.
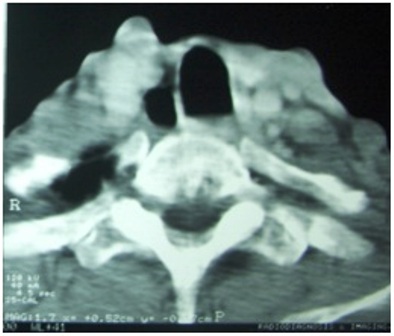
Figure 2: CT scan of the neck showing metastasis to the cervical vertebra
Patient reported back with a fungating lesion and orocutaneous fistula six months later, a surgical salvage with full thickness excision and a double flap reconstruction was planned. While the patient was being worked up for surgical excision, a chest X-ray showed evidence of pulmonary tuberculosis, that was confirmed by sputum AFB. Patient was started on anti tubercular treatment and surgery was deferred due to presence of open tuberculosis. Meanwhile, patient was started on chemotherapy with single agent oral methotrexate. A month later he presented with progressive difficulty in breathing and back pain radiating to both lower limbs. A CT scan was taken that showed multiple vertebral metastases involving dorsal and lumbar vertebrae and flared tuberculous status probably due to concomitant use of methotrexate (Figure 1,2). He died of respiratory failure 15 days after the diagnosis of vertebral metastases.
Discussion
Head and neck squamous cell carcinoma (HNSCC) is a tumor with high propensity for loco-regional spread. With newer management regimens and improved understanding of squamous cell carcinoma, loco-regional control of cancer above the clavicles has increased [7]. However, the overall disease free survival rate has not improved accordingly [23]. Distant metastases and second primary tumors are being recognized as a cause for an increasing proportion of failures in patients with head and neck malignancy [21].
Table 1: Case reports on osseous involvement in squamous cell carcinoma.
| Case reports |
Primary site |
Site of osseous involvement |
| Kerin R (1983) [27] |
3 cases of oral malignancies |
metacarpals |
Mathew BS et al., (1997)
[26] |
1 case of SCC buccal mucosa |
Vertebrae & ribs |
Carlson ER & Ord RA
(2002)33 |
4 cases of OSCC |
vertebrae |
Mendes RL et al., (2004)
[34] |
2 cases (Oral tongue & vocal cord) |
vertebrae |
| Lee KH et.al., ( 2007) [35] |
2 cases ( tongue) |
Lumbar vertebrae |
Vahtsevanos K et al., ( 2007)
[36] |
4 cases ( lip) |
Scapula, vertebrae, clavicle & ribs |
Shrivastava R et al., ( 2009)
[37] |
1 case of alveolar SCC |
Phalanges |
| Pichi B et al., (2009) [23] |
Tongue & floor of mouth |
Talus |
The reported incidence of distant metastases on autopsy is high as many distant metastases are asymptomatic and go undiagnosed [4-18]. The incidence of distant metastases varies with locations of the primary tumor in head and neck region [7,9,17,24-25]. Hsu & Chen ( 2005) [24] in their report of distant metastasis in 147 patients with HNSCC found out that the lungs were the most-common site of distant metastasis for patients with hypopharyngeal cancer and oro- pharyngeal cancer. For oral cavity cancers, the probability of metastasis to bones and lungs was equal. NPCs were more likely to metastasize to bone, followed by the liver and lungs.The most common primary sites metastasizing to distant sites are floor of mouth and tongue [25-26] and usually there are involvements at multiple distant sites [8,12,24,27-28]. Metastasis from the buccal mucosa is extremely rare and only one case report on bone metastasis from buccal mucosa had been found in our literature search [26].
Various clinical analyses have reported the correlation of occurrence of metastases with the stage of the primary tumor, the nodal involvement at diagnosis, and the development of recurrence on the primary site [24-18,29-30]. Betka (2001) [31] reported a 10% risk for stage IV disease and patients with locoregional relapse versus 3% for earlier stage disease for the development of distant metastasis. Patients with clinically palpable neck disease (N1-N3), histological evidence of nodal disease, extracapsular spread, and 3 or more positive lymph nodes are at greater risk of developing failure at distant sites [13]. De Bree et al., ( 2000) [32] reported the following risk factors for distant metastasis : three or more lymph-node metastases (almost 50% risk), bilateral lymph-node metastases, lymph nodes of 6cm or larger, low jugular lymph-node metastases, locoregional tumour recurrence, and second primary tumour. The incidence of distant metastasis has been found to be higher in patients with locoregional failure than in those with loco-regional control and 21.4% in patients with loco-regional recurrence [15,24,33].
In a limited literature review that was carried out, we came across several case reports on osseous metastatic involvement from OSCC and HNSCC (Table 1) [26,27,34-38] and retrospective analyses which reported on the incidence, sites of involvement and probable predictors of distant metastases from OSCC and HNSCC are detailed in Table 2. [6-9,11,12,20,22,24,25,29,39-44].
Table 2 : Case series on incidence of distant metastases and bone involvement.
| Author & year |
Incidence of distant metastases |
Incidence of bone metastases |
| Hoye RC et al., ( 1962) [38] |
55% (23/42) |
39.1% (9/23) |
| Ju DMC ( 1964) [39] |
52% (152 /293) |
35.5% (54/152) |
| O’Brien PH et al., ( 1971) [25] |
46.7% ( 57/122) |
23% (13 /57) |
| Probert et al., ( 1974) [8] |
12.3% ( 96/ 779) |
25% ( 24/96) |
| Merino OR et al., ( 1977) [6] |
10.9% (546/5019) |
20.3% (110/546) |
| Dennington ML et al., ( 1980) [40] |
40% (25/64) |
24% (6/25) |
| Vikram B et al., ( 1984) [7] |
17.5% (20/114) |
35% (7/20) |
| Papac RJ et al., ( 1984) [9] |
30.7% ( 52/ 169) |
44.2% ( 23/52) |
| Zbaren & Lehmann ( 1987) [11] |
40% (40 /101) |
15% ( 6/40) |
| Kotwall C et al., ( 1987) [12] |
47% ( 387/ 832) |
30% ( 118/387) |
| Leeemans CR et al.,( 1993) [29] |
9.3% (26/281) |
30.7 % (8/26) |
| Calhoun FH et al., ( 1994) [20] |
11.4% ( 83/727) |
31.1% (26 /83) |
| Wollenberg, B., et al., ( 1994) [41] |
|
37% ( 41/108) |
| Troell RJ,& Terris DJ (1995) [42] |
14.4% (14/97) |
35.7% (5/14) |
| Shingaki S et al.,( 1996) [30] |
20% (21/103) |
23.8%(5/21) |
| Hsu LP & Chen PR ( 2005) [24] |
12% (35/291) |
51.4% (18/35) |
| Garavello et al., ( 2006) [43] |
9.2%( 181/1972) |
9.9% ( 18/ 181) |
| Basu et al., ( 2007) [22] |
|
1.9% (13/683) |
These studies have shown the bone to be the second common site of distant spread, yet the incidence is much lower when compared with pulmonary involvement. Because of the relatively low frequency of bone metastases, screening for distant metastases at sites other than the lungs is usually not carried out. For oral squamous cell carcinoma, bone metastases traditionally have only come to attention through pain and symptoms of pathologic fractures, or abnormal laboratory test results, all of which are insensitive for early lesions [22]. Vertebral involvement in our patient was suspected because of severe back pain radiating to lower limbs.
Bone scintigraphy has not yet become a routine screening tool, and is carried out only in cases where there is high suspicion of osseous involvement. There are mixed reports of the usefulness of scintigraphy. Belson (1980) [45] reported positive bone scintigraphy in 2% of patients while Ampil et al, (1995) [46] could not detect any case on screening bone scans of 93 patients with locally advanced head and neck cancer.
The reported frequencies of bony metastasis vary from 11 to 54%, but with loco-regional control of disease, the incidence decreases considerably [15,18,22,24].The osseous distant metastases have been commonly reported in vertebrae, ribs, long bones, ilium, clavicles, and skull [7-8,19,26,34-37,40,47-49]. Usually multiple bones are involved [8,25,48].Rarely phalanges and talus have been involved [22,27,37,47]. Major symptoms of osseous metastasis are related to pain and pathologic fractures that direct attention to metastatic foci. In cases of vertebral involvement, spinal cord compression has been reported [34-37]. Our patient suffered from spinal cord compression due to involvement of lumbar and dorsal spines.
Carlson & Ord (2002) [34] reported a 0.7% incidence of vertebral metastasis in 597 patients with OSCC. All patients reported with stage IV disease of gingival or retromolar area. Basu et al., ( 2007) [22] reported bone metastasis in 13 patients with HNSCC. All of the patients had advanced-stage disease at initial presentation, and most developed bone metastases early in the post therapeutic course. 4 cases ( 30.7%) were SCC of base of tongue and one was from oral tongue.
Most studies found that distant metastases are diagnosed within a year of presentation [9-10] so there is probably subclinical seeding of malignant cells before the eradication of the primary tumor. The average survival with distant metastasis ranges between 4.3 months and 7.3 months [9,11-12]. Our patient developed vertebral involvement after 7 months of primary treatment.
Conclusion
Squamous cell carcinoma of the head and neck region tends to spread by direct extension and lymphatic metastasis, with hematogenous dissemination occurring late in the natural history of the disease. The distant metastases can affect different organ systems and almost invariably herald a poor prognosis and treatment is usually palliative. Constant vigil is required to detect the metastases early and any unexplained bony pain or functional deficits should alert the clinicians to the probability of bone metastases, as these may be the only symptoms of bone involvement.
References
[1]. Mahomed F, Altini M, Meer S. Altered E-cadherin/ß-catenin expression in oral squamous carcinoma with and without nodal metastasis. Oral Dis. 2007;13:386-92
[2]. Crile G: Excision of cancer of the head and neck: With special reference to the plan of dissection based on one hundred and thirty-two operations. J Am Med Assoc . 1906; 47:1780
[3]. Crile GW. Carcinoma of the jaws, tongue, cheek, and lips. Surg. Gynecol. Obstet. 1923; 36: 159-184.
[4]. Arons MS, Smith RR. Distant metastases and local recurrence in head and neck cancer. Ann Surg 1961; 154: 235-40.
[5]. Rubenfeld S, Kaplan G, Holder AA. Distant metastases from head and neck cancer. AJR 1962; 87: 441-8.
[6]. Merino OR, Lindberg RD, Fletcher GH. An analysis of distant metastasis from squamous carcinoma of upper respiratory and digestive tracts. Cancer 1977; 40: 145- 151.
[7]. Vikram B, Strong EW, Shah JP, Spiro R. Failure at distant sites following multimodality treatment for advanced head and neck cancer. Head Neck Surg 1984; 6: 730–733.
[8]. Probert JC, Thompson RW, Bagshaw MA: Patterns of spread of distant metastases in head and neck cancer. Cancer 33:127, 1974
[9]. Papac RJ. Distant metastases from head and neck cancer. Cancer1984;53:342- 345.
[10]. Shaha AR, Spiro RH, Shah JP, Strong EW: Squamous carcinoma of the floor of the mouth. Am J Surg 1984; 148: 455-459.
[11]. Zbaren P, Lehmann W: Frequency and sites of distant metastases in head and neck squamous cell carcinoma: An analysis of 101 cases at autopsy. Arch Otolaryngol Head Neck Surg 113:762, 1987
[12]. Kotwall C, Sako K, Razack MS, et al: Metastatic patterns in squamous cell cancer of the head and neck. Am J Surg154:439, 1987
[13]. Alvi A, Johnson JT. Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck 1997; 19: 500 – 505.
[14]. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE: Distant lymphatic metastasis from head neck cancer. Ann Otol Rhinol Laryngol 1999;108:860–863.
[15]. Leon X, Quer M, Orus C, et al. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680–686.
[16]. Ferlito A, Shaha A, Silver CE, et al. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec 2001;63:202- 207
[17]. Awwad HK, Lotayef M, Shouman T, Begg AC, Wilson G, Bentzen SM, El- Moneim HA, Eissa S. Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: influence of proliferation. British Journal of Cancer (2002) 86, 517 – 523
[18]. Ljumanovic R, Langendijk JA, Hoekstra OS, Leemans CR, Castelijns JA. Distant metastases in head and neck carcinoma: Identification of prognostic groups with MR imaging .European Journal of Radiology 2006; 60: 58–66.
[19]. Gowen GF, de Suto-Nagy G. The incidence and sites of distant metastases in head and neck carcinoma. Surg Gynecol Obstet 1963; 116: 603–607.
[20]. Calhoun FH, Fulmer P, Weiss R, Hokanson JA. Distant metastases from head and neck squamous cell carcinomas. Laryngoscope 1994; 104: 1199–1205
[21]. Talmi YP, Cotlear D, Waller A et al. Distant metastases in terminal head and neck cancer patients. J Laryngol Otol 1997; 111: 454–458.
[22]. Basu D, Siegel BA, Mc Donald DJ et al. Detection of occult bone metastases from head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 2007;133: 801 – 805.
[23]. Pichi B, Marchesi P, Manciocco V, Ruscito P, Pellini R, Cristalli G, Terenzi V, Spriano G. Carcinoma of the buccal mucosa metastasizing to the talus. J Craniofac Surg 2009;20: 1142 – 1145
[24]. Hsu LP, Chen PR. Distant metastases of head and neck squamous cell carcinomas — Experience from eastern Taiwan. Tzu Chi Med J 2005; 17:99-104.
[25]. O’Brien PH, Carlson K, Steubner EA, Staley CT. Distant metastases in epidermoid cell carcinoma of the head and neck. Cancer. 1971;27: 304-307 .
[26]. Mathew BS, Jayasree K, Madhavan J, et al. Skeletal metastases and bone marrow infiltration from squamous cell carcinoma of the buccal mucosa. Oral Oncology 1997;33:454 - 455
[27]. Kerin R. Metastatic tumours of the hand. A review of literature. J Bone Joint Surg Am. 1983; 65: 1331-1335
[28]. Richey LM, Shores CG, George J, Lee S, Couch MJ, Sutton DK, Weissler MC. The effectiveness of salvage surgery after the failure of primary concomitant chemoradiation in head and neck cancer. Head Neck Surg. 2007; 136: 98 – 103.
[29]. Leeemans CR, Tiwari R, nauta JJP, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993; 71(2): 452.
[30]. Shingaki S, Suzuki I, Kobayashi T, Nakajima T. Predicting factors for distant metastases in head and neck carcinomas: An analysis of 103 patients with locoregional control. J Oral Maxillofac Surg. 1996; 54:853-857
[31]. Betka J. Distant metastases from lip and oral cavity cancer. ORL J Otorhinolaryngol Relat Spec 2001: 63: 217–221.
[32]. de Bree R, Deurloo EE, Snow GS, Leemans CR. Screening for distant metastases in patients with head and neck cancer. Laryngoscope 2000: 110: 397–401.
[33]. Liao CT, Wang HM, Chang JTC, Ng SH, Hsueh C, Lee LY, Lin CH,Chen IH, Huang SF, Yen TC..Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer 2007;110:1501–1508.
[34]. Carlson ER, Ord RA. Vertebral Metastases From Oral Squamous Cell Carcinoma. J Oral Maxillofac Surg 2002; 60:858-862.
[35]. Mendes RL, Nutting CM, Harrington KJ. Residual or recurrent head and neck cancer presenting with nerve root compression affecting the upper limbs. Br J Radiol 2004;77:688–90.
[36]. Lee KH, Halfpenny W, Thiruchelvam JK. Spinal cord compression in patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103:e16–8.
[37]. Vahtsevanos K, Ntomouchtsis A, Andreadis C, et al. Distant bone metastases from carcinoma of the lip: a report of four cases. Int J Oral Maxillofac Surg 2007; 36: 180 – 185.
[38]. Shrivastava R, Singh KK, Umbarker BR, Karle R, Shrivastava M. Phalange metastasis from carcinoma of alveolus . Ind J Dent Res. 2009; 20(4): 496-498.
[39]. Hoye RC, Herrold KM, Smith RR, Thomas LB. A clinicopathological study of epidermoid carcinoma of the head and neck. Cancer. 1962; 15: 741-749.
[40]. Ju DMC. A study of the behavior of cancer of the head and neck during its late and terminal phases. Am J Surg. 1964; 108: 552-557.
[41]. Dennington ML, Carter DR, Meyers AD. Distant metastases in head and neck epidermoid carcinoma. Laryngoscope 1980;90:196 -201
[42]. Wollenberg, B., Ollesch, A., Maag, K., et al., Micrometastases in bone marrow of patients with cancers in the head and neck area. Laryngorhinootologic, 1994, 73(2), 88-93
[43]. Troell RJ, Terris DJ. Detection of metastases from head and neck cancers. Laryngoscope. 1995; 105(3): 247-250.
[44]. Garavello W, Ciardo A, Spreafico R, Gaini RM. Risk Factors for Distant Metastases in Head and Neck Squamous Cell Carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:762-766
[45]. Belson TP. Bone and liver scans in patients with head and neck carcinoma. Laryngoscope, 1980; 90:1291-1296.
[46]. Ampil FL, Wood M.J, Chin H W., et al., Screening bone scintigraphy in the staging of locally advanced head and neck cancer. J Cran maxfac surg, 1995, 23(2), 115-118.
[47]. Bloodgood JC. Bone tumors, benign and malignant: a brief summary of the salient features, based on a study of some 370 cases. Am J Surg 1920;34:229.
[48]. Arlen M, Wanebo H, Guerra O, Higinbotham N, Huvos A, Miller T. Osseous Metastasis: Its Relationship to Primary Carcinoma of the Head and Neck . Am J Surg. 1974;128: 568 -572.
[49]. Ildstad ST, Bigelow ME, Remensnyder JP. Intra-oral cancer at the Massachusetts general hospital : squamous cell carcinoma of the floor of the mouth. Ann. Surg. 1983; 197(1): 34- 41.


