
Maxillectomy for Carcinoma in Young Adults: A Retrospective Analysis
Elizabeth Mathew Iype1, Anmol Agarwal1, Preethi Sara George2.
- 1Departments of Surgical Oncology. Regional Cancer Centre, Trivandrum, India-695011
- 2Departments of Epidemiology. Regional Cancer Centre, Trivandrum, India-695011
- Submitted: March 10, 2012;
- Accepted July 7, 2012
- Published: August 9, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Introduction:
Maxillectomy is required for the tumors arising in maxillary sinus, hard palate, upper alveolus or lateral aspect of the nasal cavity. The purpose of this paper is to present the experience in young adults undergoing maxillectomy for malignancy.
Aims & Objectives:
To study the histopathological profile and primary site distribution of the malignant tumors in young adults undergoing maxillectomy and also to study the factors influencing the survival after primary and salvage maxillectomy for malignancy.
Materials & Methods:
A retrospective analysis of young adults (18- 45 years) who underwent maxillectomy for carcinoma of the upper alveolus, maxillary sinus, hard palate or nasal cavity treated at a Cancer Center between 2002-2006.
Results:
Hard palate & Upper alveolus were found to be the commonest site of tumor in the young patients. Squamous cell carcinoma was the major histological diagnosis. On multivariate analysis male patients and patients in 35- 45 year age group had a significantly better survival compared to females and patients
< 35 years. The node positive cases had a significant poor survival compared to node negative cases and those treated with total maxillectomy showed a significantly better survival rate.
Conclusion:
The patients in whom maxillectomy was done as a primary modality of treatment had better survival compared to salvage surgery patients although it was not significant statistically. The nodal status of these patients was a statistically significant factor adversely influencing the disease free survival.
Introduction
Maxillectomy is required for the tumors arising in maxillary sinus, hard palate, upper alveolus or lateral aspect of the nasal cavity and rarely for tumors arising from the neighboring sites like ethmoid sinus or orbit. Surgical resection alone or more commonly combined with adjunctive therapy remains the mainstay of treatment for tumors in these areas [1]. The histopathological variants of tumors seen here are squamous cell carcinoma, minor common salivary gland tumors like adenoid cystic carcinoma, adenocarcinoma, mucoepidermoid carcinoma and rarely sarcoma [2].
The purpose of this paper is to present the experience in young adults undergoing maxillectomy for malignancy of the maxillary sinus, hard palate, upper alveolus and the nasal cavity
Materials & Methods
A retrospective analysis of 27 young adults (18-45 years) who underwent maxillectomy for carcinoma of the upper alveolus, maxillary sinus and hard palate or nasal cavity at the Regional Cancer Center, Trivandrum, India between 2002-2006 was done. The subjects were followed up for a median period of 41 months (range 1-98 months) the end points of interest were disease-free survival time. Fisher’s exact, or the test was used to find risk factors for surgical adverse effects and poor survival. Comparisons of survival were made with the Kaplan-Meier method and log-rank test.
Results
Out of 27 patients, 14 were males and 13 females. The mean age was 31 years (range 18 – 45 years). Hard palate & Upper alveolus were found to be the commonest site of tumor in the patients included in this study. Forty eight percentage of the patients had early tumors (T & T2) and rest had late tumors (T3 & T4). Minor salivary gland tumors were found to be the commonest histopathological variant 15/27(55.5%). Squamous cell carcinoma constituted 37% cases (10/27). The majority of maxillary sinus tumors were squamous cell carcinoma (77.7%). The majority of palatal /alveolar tumors were minor salivary gland tumors (88.2 %). The sole case of nasal cavity tumor was mucoepidermoid carcinoma. Nodal disease was seen in 3 patients (21.2%).
All the 27 patients were treated with a curative intent. Maxillectomy was done as primary procedure in 20 patients (74%) and as salvage in 7 (26%) patients. The procedure was performed via a Weber Fergusson’s approach for majority of cases (70 %) and in rest 30 %, transoral approach was used. Amongst all, 10 patients (37%) were treated with surgery only, 10 (37%) by surgery followed by postoperative radiotherapy and rest 7 (26%) were treated by radiation therapy followed by surgery. Surgery consisted of 12 (44.4%) partial maxillectomy, 6 (22.2%) subtotal maxillectomy, 2 (7.4%) total maxillectomy, 6 (22.2%) radical maxillectomy with orbital exenteration, and 1(3.8%) bilateral maxillectomy. Neck dissection was done in 3 patients.
Among the 27 patients 21 are free of disease till date and recurrence was seen in 6 patients (22.2%) in the primary site, most commonly in the hard palate or upper alveolus. Failure in the regional lymph nodes was observed in 2 (7.4%). The recurrence pattern is shown in the table below. Only 2 of these patients could be salvaged. The overall disease free 5 year survival rate of the 27 patients was 69.5%. The group of patients who underwent preoperative RT followed by surgery was the largest group who had a 5 year survival rate 80%. According to the Multivariate analysis males, patients 35- 45 years of age, patients treated with total maxillectomy showed a better survival rate though statistically not significant. The 3 year disease free survival for the salivary gland tumors was 88% compared to 77% for the squamous cell carcinoma which was statistically significant (Figure 1). The 3 year survival for the node negative and the node positive cases were 84% and 50% respectively which was also statistically significant. (Figure 2)
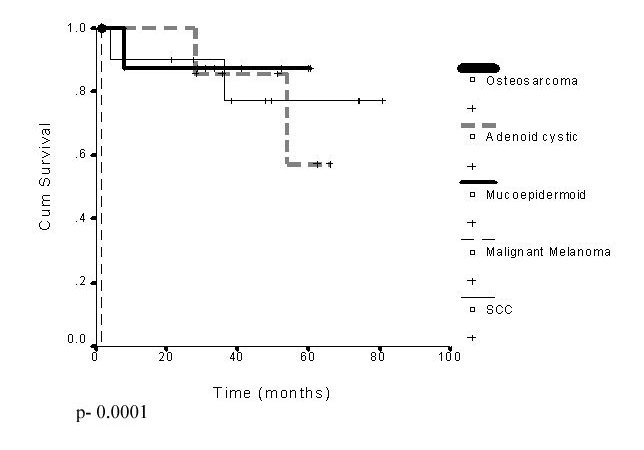
Figure 1: Disease free survival
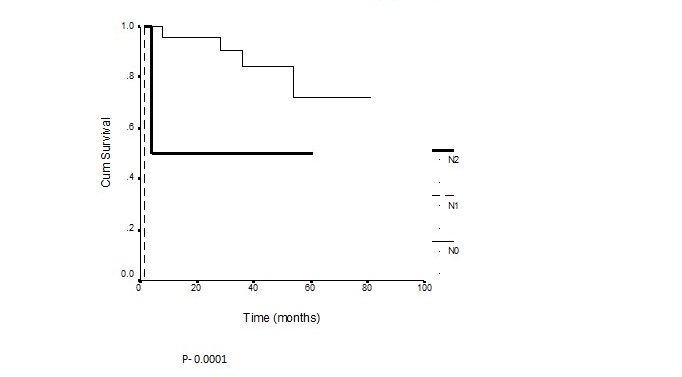
Figure 2: Disease free survival by N Stage
Table 1: Distribution of cases
| Variable |
Total Patients
(n=27) |
Patients with
recurrence
(n=6) |
Patients with out
recurrence
(n=21) |
Five year
survival rate
(%) |
Pvalue* |
Age <=35 years
>35 years |
16
11 |
4 (25%)
2 (18.2%) |
12(75%)
9(81.8%) |
66.33%
81.82% |
0.9146 |
sex Male
Female |
14
13 |
2 (14.3%)
4 (30.7%) |
12 (85.7%)
9 (69.3%) |
80.77
63.46 |
0.4802 |
Site Maxilla
Nasal cavity
Palate/ Upper alveolus |
9
1
17 |
1 ( 11.1%)
1 (100 %)
4 (23.5%) |
8 (88.9%)
0
13 (76.5%) |
70.05
0
85.5 |
0.3365 |
T stage T1 & T2
T3 & T4 |
13
14 |
1 (7.6%)
5 ( 35.7%) |
12 (92.4%)
9 (64.3%) |
82.65
65.46 |
0.0224 |
N Stage No
N+ |
24
3 |
4 (16.6%)
2 (66.7%) |
20 (83.4%)
1 (33.3%) |
72.32
33.33 |
0.0197 |
Type of Surgery
Primary
Salvage |
20
7 |
3 (16%)
2 (28.5%) |
17 (84%)
5 (61.5%) |
75.26
60.35 |
0.9601 |
Extend of surgery Partial
Subtotal
Total
Radical
Bilateral |
12
6
2
6
1 |
3(25%)
0
2(100%)
1(16.7s%)
0 |
9(75%)
6(100%)
0
5(83.3%)
1(100%) |
75.0
8303
0
0
N.C |
0.6937 |
Histopathology
SCC
MM
MEC
ACC
SAR |
10
1
7
8
1 |
2(20%)
1(100%)
1(14.3%)
2(25%)
0 |
8(80%)
0
6(83.7%)
6(75%)
1(100%) |
74.07
0
83.3
57.1
N.C |
0.0001 |
Data are expressed as numbers and percentages of patients. Because of rounding, percentages may not sum to 100.
*Comparison of patients with recurrence versus patients without recurrence.
SCC: Squamous cell carcinoma, MM: Malignant melanoma, MEC: Mucoepidermoid carcinoma, ACC: Adenoid cystic carcinoma
SAR: Sarcoma
Discussion
The prognosis for the sinonasal malignancies has remained poor for the past several decades despite of improvements in both surgical techniques and adjuvant therapies. Stern reviewed Anderson's experience for maxillary squamous cell carcinoma and found no significant improvement in survival when compared to a similar study, 20 years earlier [3]. Surgery with postoperative radiation therapy remains the standard treatment for resectable sinonasal carcinomas.
Spiro et al., reviewed 105 patients at Memorial Sloan-Kettering Cancer Center with nasal cavity, maxillary, and ethmoid squamous cell carcinoma treated with combination surgery and radiation therapy, radiation therapy alone, or surgery alone [4]. The majority of these patients presented with extensive disease with 82 percent of newly treated patients having stage III or stage IV disease. Survival correlated to the stage at presentation, and the overall 5-year survival was 37 percent. The survival rates for nasal, maxillary, and ethmoid tumors were 45, 38, and 13 percent, respectively. The local control for maxillary sinus tumors was 49 percent, and local recurrence was the most common site of failure.
Zaharia et al., reported the outcome of 149 patients treated with surgery and postoperative radiation therapy for a variety of malignant histologies. The 5-year actuarial survival was 36.2 percent overall, while for squamous cell carcinoma alone it was 35 percent [5]. In the same year a 49 percent 5-year survival after treatment of 60 patients with sinonasal malignancies, with a variety of regimens, was described [6].
An analysis of 73 patients with maxillary sinus malignancies of varying histologies, treated with surgery and postoperative radiation therapy, gave the overall 5-year relapse free survival 51 percent, with a local control rate of 78 percent [7].
The 10-year Cleveland Clinic experience comprises 54 patients with squamous cell carcinoma of the sinuses: all received surgery and/or radiation therapy with an overall survival of 38.2 percent for the maxillary sinus group [8].
Among the 27 patients analyzed in the present study, minor salivary gland tumors were the commonest histopathological variant followed by squamous cell carcinoma. The majority of maxillary sinus tumors were squamous cell carcinoma and the majority of palatal /alveolar tumors were minor salivary gland tumors. Maxillectomy was done as primary procedure in almost three fourths of patients. Maxillectomy was done via a Weber Fergusson’s approach for majority of cases. Among the 27 patients 21 are free of disease at present and local recurrence was seen in 6 patients (22%).The overall treatment of maxillary malignancies in our group has resulted in 5-year survival rates in the 69.5% percent range.
Similar results were achieved at the department of Otolaryngology VU Amsterdam where 43 patients with squamous cell carcinoma were treated from 1975 to 1994 [9]. Eighty-three percent of the tumors were in stage III or stage IV at the time of first presentation. Five-year survival after surgery and postoperative radiotherapy for all patients was 64%. For stages II, III, and IV it was 83, 49, and 37 percent, respectively [9].
Factors affecting survival in maxillary sinus cancer were analyzed in a large study (650 patients with maxillary sinus cancer) from Brigham and Women's Hospital in Boston [10]. The overall mean (median) survival was 52 months (25 months). A high percentage of patients, 77.5 and 7.4 percent of patients presented with advanced (T3/T4) disease or cervical metastasis, respectively. Survival for patients with maxillary sinus cancer was determined not only by TNM staging but also by tumor histology and grade. TNM staging effectively stratifies patients according to survival. Radiation therapy significantly improves survival for those with T4 lesions.
Treating maxillary sinus cancer is challenging because of the proximity of critical structures, such as the eye and the brain, which preclude wide surgical excision and high-dose radiotherapy. The clinical course is indolent at most and a substantial number of patients have advanced disease at the time of diagnosis.
Combined-modality therapy consisting of surgery and radiotherapy with or without intraarterial chemotherapy is generally used for the treatment. The reported 5-year local control and survival rates are 50-78 and 39-64 percent, respectively [11-13] which is very much similar to what has been seen in the present study. However, an appropriate treatment strategy in terms of surgical procedure, radiotherapy methods and their sequence is still a matter of controversy.
Local control is a particularly difficult problem, with the majority of failures occurring at the primary site. These difficulties with maxillary cancer treatment are linked to the complex anatomy of the paranasal sinus region, and a propensity for late presentation due to the absence of symptoms in an early stage of disease. Complete surgical removal of the tumor with postoperative radiation therapy remains the standard of care for resectable lesions. Improved reconstructive techniques including microvascular free flaps and prosthetic obturators have significantly decreased the functional and cosmetic morbidity from aggressive surgical resection.
Conclusions
The patients in whom maxillectomy was done as a primary modality of treatment had better survival compared to patients in whom it was done as a salvage procedure although it was not significant statistically. The nodal status of these patients was a statistically significant factor adversely influencing the disease free survival.
Authors' Contribution
EMI: Conceived and designed the study and prepared the manuscript.
AA: Conducted the literature review and helped in preparation of manuscript.
PSG: Conducted the statistical analysis and contributed to the manuscript preparation.
Conflict Of Interest
The authors declare that there are no conflicts of interests.
Ethical Considerations
Retrospective chart review, exempted from ethical committee review.
Funding
None
Acknowledgement
None
References
[1]. Chang Choi E, Choi Y, Kim K, et al. Surgical outcome of radical maxillectomy in advanced maxillary sinus cancers. Yonsei Med J 2004; 45:261–8.[Pubmed]
[2]. Midion Mapfumo Chidzonga, Leonard Mahomva. Squamous cell carcinoma of the oral cavity, maxillary antrum and lip in a Zimbabwean population: A descriptive epidemiological study. Oral Oncology, 2005:23,184-187[Pubmed]
[3]. Stern SJ, Goepfert H, Clayman G, et al. Squamous cell carcinoma of the maxillary sinus. Arch Otolaryngol Head Neck Surg 1993; 119:964-9.[Pubmed]
[4]. Spiro JD, Soo KC, Spiro RH. Non squamous cell malignant neoplasms of the nasal cavities and paranasal sinuses. Head Neck l995; 17:114-118. [Pubmed]
[5]. Zaharia, M, Salem LE, Travezan R, et al. Postoperative radiotherapy in the management of cancer of the maxłłlary sinus. Int J Radiat Oncol Biol Phys 1989; 17:967-971. [Pubmed]
[6]. Sisson GA, Toriumi DM, Atiyah RA. Paranasal sinus malignancy: a comprehensive update. Laryngoscope 1989;99: 143-150[Pubmed]
[7]. Jiang GL, Ang KK, Peters LJ, et al. Maxillary sinus carcinomas: natural history and results of postoperative radiotherapy. Radiother Oncol 1991; 21:193-200.[Pubmed]
[8]. Lavertu P, Roberts JK, Kraus DH, et al. Squamous cell carcinoma of the paranasal sinuses: the Cleveland Clinic experience 1977-1986. Laryngoscope 1989; 99:1130-1136.[Pubmed]
[9]. Tiwari R, Hardillo JA, Mehta D, et al. Squamous cell carcinoma of maxillary sinus. Head Neck 2000; 22:164-169.[Pubmed]
[10]. Bhattacharyya N. Factor affecting survival in maxillary sinus cancer. J Oral Maxillofac Surg 2003; 61:1016-1021. [Pubmed]
[11]. Paulino AC, Marks JE, Bricker P, Melian E, Reddy SP, Emami B. Results of treatment of patients with maxillary sinus carcinoma. Cancer 1998; 83:457-465.[Pubmed]
[12]. Sakat K, Aoki Y, Karasawa K, Nakagawa K, Hasezava K, Muta N. Analysis of the results of combined therapy for maxillary carcinoma. Cancer 1993; 71:2715-2722.[Pubmed]
[13]. Itami J, Uno T, Aruga M, Ode S. Squamous cell carcinoma of the maxillary sinus treated with radiation therapy and conservative surgery. Cancer 1998; 82:104-107.[Pubmed]


