Case Report

A case of young breast cancer in which delivery was successfully achieved after postoperative chemotherapy under ovarian function protection using GnRH agonist
1Shinichiro Kashiwagi, 1Tsutomu Takashima, 1Naoki Aomatsu, 1Hidemi Kawajiri, 1Naoyoshi Onoda, 1Tetsuro Ishikawa, 1Kosei Hirakawa
- 1Department of Surgical Oncology, Osaka City University Graduate School of Medicine, 1-4-3 Asahi-machi, Abeno-ku, Osaka, Japan.
- Submitted: August 11, 2012,
- Accepted September 16, 2012
- Published: October 15, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Introduction
Breast cancer occasionally occurs in reproductive period. These patients tend to treat with intensive adjuvant or neoadjuvant chemotherapy because of their younger age if cytotoxic chemotherapy is indicated. However, cytotoxic chemotherapy may cause loss of fertility. There are several reports suggesting possibility of ovarian function preservation by administration of GnRH agonist concomitant with cytotoxic chemotherapy.
Case report
Herein we report a case of spontaneous delivery after adjuvant chemotherapy using FEC*100 followed by weekly paclitaxel under ovarian protection by GnRH agonist. A 28-year-old woman received a mastectomy due to a right breast cancer. Adjuvant chemotherapy was recommended because of high nuclear grade, younger age and negative hormonal receptor status. However, the patient had the strong hope of pregnancy. GnRH agonist was started prior to chemotherapy due to preventing loss of ovarian function by cytotoxic agents. Four cycles of FEC*100 every 3 weeks followed by 12 cycles of paclitaxel every week with GnRH agonist every 4 weeks was administered. Menstruation was recovered 4 months after ending of chemotherapy. She got pregnant spontaneously 15 months after ending of chemotherapy. And she delivered a 2,825g healthy baby in 40weeks 3days.
Conclusions
Fertility may be preserved by ovarian suppression with GnRH agonist during cytotoxic chemotherapy.
Key words
young breast cancer, protection of ovarian function, GnRH agonist.
Introduction
In treating young breast cancer patients, damage to the ovarian function due to chemotherapy markedly affects the patients, causing chemotherapy-induced amenorrhea (CIA), a decrease in the rate of menorrhea recovery, and early menopause [1,2]. Chemotherapy damages ovarian function mainly involving primary and secondary follicles, and immature follicles are relatively preserved. CIA is directly associated with infertility, and can be a significant problem in chemotherapy for breast cancer, in which a large number of patients are premenopausal females. Recently, the protection of ovarian function (POF) by the combined use of a GnRH agonist with chemotherapy has been suggested as a way to prevent CIA [3-5]. The GnRH agonist has been used as an endocrine therapy for hormone receptor positive premenopausal breast cancer patients. It suppresses estrogen by temporarily increasing the secretion of gonadotropic hormones from the pituitary gland, overstimulating the ovaries, resulting in down regulation of the ovarian function by including negative feedback from the ovaries [6]. By suppressing oocyte development, immature follicles become predominant, and the risk of CIA development can be reduced.
Based on these theories, if chemotherapy is conducted with POF to suppress the ovarian function, the rate of CIA development may be reduced. In animal experiments, the effect of GnRH agonists on reducing the ovarian toxicity of cyclophosphamide has been verified [6]. Clinically, some cases have been reported in Western countries in which normal pregnancy and delivery were achieved after chemotherapy combined with GnRH agonist use [4].
Herein, we encountered a case of young breast cancer in which pregnancy and delivery were successfully achieved with the use of POF. We present the case with a review of the literature.
Case Report
A 28-year-old woman presented with mass in her right breast. The past, family and medical history was not contributory. She visited a local clinic due to a lump in the right breast. Ultrasonography showed a hypoechoic tumor suspected a fibroadenoma. Excisional biopsy was performed under local anesthesia to confirm the diagnosis. Pathological examination showed infiltrating ductal carcinoma, and then she was referred to our hospital for additional treatment.
At admission her height was 146 cm and weight 39 kg . She got married at the age of 23, menarche at the age of 11, was premenopausal, gravida 1, and miscarriage 1. Physical examination revealed a 3cm length surgical wound in the lower lateral quadrant region of the right breast. There were no palpable tumors, no discharge from the nipple, and no palpable axillary lymph nodes. There were no abnormal blood biochemical findings. There was no elevation in tumor markers. Mammography showed a tumor shadow with lobulated shape and well-defined smooth margin tumor was observed in the left breast (Figure 1). On Ultrasonography a 10.0 x 8.6 x 5.7 mm sized hypoechooic lenticular shaped tumor with smooth margin was observed in the lateral region of the left breast. Histopathological examination of a tumor specimen obtained by tumor extirpation performing at local clinic revealed infiltrating ductal carcinoma, ER (-), PR (-), HER2 (-), the histological grade 3 by Bloom and Richardson classification and positive margin.
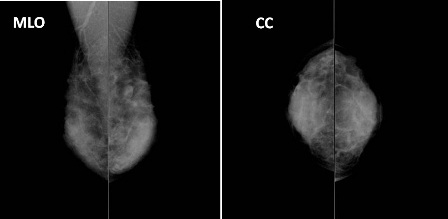
Figure 1. Findings of mammography. Heterogeneous with a high density. A lobulated, well- and smoothly-defined, and isoconcentrated tumor was observed in the left breast.
Surgery was performed prior to chemotherapy, as the tumor had removed already and clinically node negative. Postoperative chemotherapy was considered to be essential as the case belonged to a high risk group with triple negatives, the age was lower than 35, with a histological grade 3. A Nipple-preserving subcutaneous total mastectomy with axillary lymph node dissection, and reconstruction using the latissimus dorsi musucular flap were performed. Pathological examination showed the tumor was solid-tubular carcinoma, g, ly0, v0, nuclear grade 3. Immunohistochemistry showed laveling index of estrogen receptor and progesteron receptor were both 0% and HER2 score was estimated as 1+ (Figure 2).
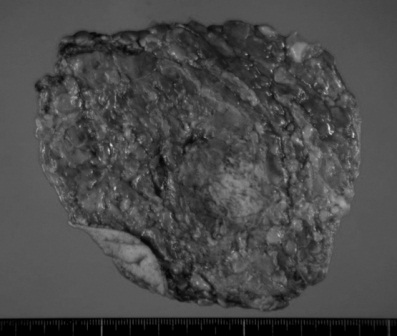
Figure 2. Excised specimen and pathological examination. Solid-tubular carcinoma, g, ly0, v0, n(0/10), nuclear grade 3 (score 5), nuclear atypia 3, mitotic counts 3, and ER / PR / HER2=0% / 0% / 1+.
Based on the risk category of recurrence proposed by the consensus panel of St. Gallen 2007, this case belonged to the high risk group with the features of hormone receptor negatives, an age of less than 35, and nuclear grade of 3. Postoperative chemotherapy including 4 cycles of FEC*100 and 12 cycles of weekly paclitaxel was scheduled (Figure 3). She desired to have a child, and so GnRH agonist was administered to protect the ovarian function, although the hormone receptors were negative (Figure 4). After the chemotherapy, she received regular follow-up at the outpatient clinic every 3 months and appeared no evidence of recurrence. Pregnancy was confirmed 15 months after completion of chemotherapy. The pregnancy had maintained without major complication. She delivered a 2,825g healthy girl, APGAR score 9 at 40weeks 3days of gestation. The baby has been growing well without any abnormality.
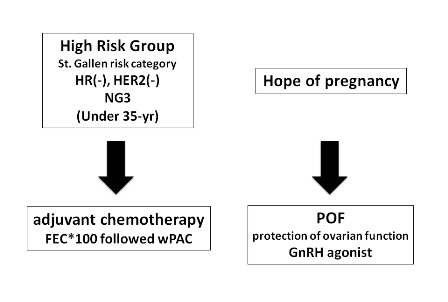
Figure 3. Postoperative plan. Based on the St. Gallen risk category, this case belonged to the high risk factor group, and so adjuvant chemotherapy was conducted. She desired to have a child, and so GnRH agonist was administered to protect the ovarian function, although the hormone receptors were negative.
Discussion
Young breast cancer patient is generally defined as developing in patients less than 35 years old. They occupied 5-10 % of whole breast cancer patients, known to be high recurrence rate and to have a poor prognosis [7-10]. In Japan, the rate of no-gravida in patients of young breast cancer is increasing due to social structure changes, such as a tendency of late marriage and late delivery. As the number of no-gravida patient increases, the rate of patients who desire to have a child is also increasing. In the risk category proposed by St. Gallen consensus meeting before 2009, age lower than 35 was one of the risk factors, and such cases were recommended to undergo chemotherapy [7]. However, the indication of chemotherapy has revised at St. Gallen consensus meeting in 2009 and the condition of age lower than 35 became to be omitted from the risk factors indicating chemotherapy.
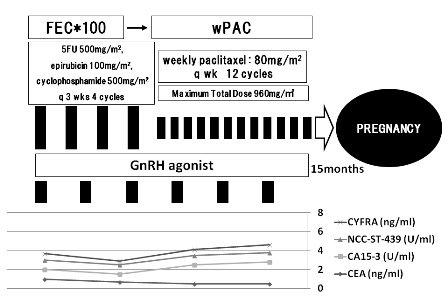
Figure 4. Postoperative course. Postoperative chemotherapy was conducted involving 4 cycles of FEC*100 and 12 cycles of weekly paclitaxel with GnRH agonist. After the chemotherapy, she was monitored at the outpatient clinic, with no metastasis or recurrence. Pregnancy was confirmed 15 months later.
CIA is defined as amenorrhea lasting for more than 3 months occurring within one year after the start of chemotherapy [1]. The rate of CIA is 94, 58.8, and 53 - 68.8% with cyclophosphamide, paclitaxel, and CMF, respectively [2]. There is a strong possibility that combination therapy using GnRH agonist reduces the occurrence of CIA and it may protect the ovarian function. In Western countries, there are some reports on pregnancy and normal delivery in cases in which GnRH agonist was used concurrently with chemotherapy [4], and clinical trials such as the ZORO study and SWOG-S0230 are being conducted. However, ASCO’s guideline for fertility preservation and Japanese guidelines for breast cancer do not recommend the use of a GnRH agonist for the purpose of preventing CIA, because there are few sufficient evidence supporting its safety and effect.
Early menopause caused by chemotherapy is a favorable condition for young patients with ER-positive breast cancer. However, the risks and benefits in the conccurent use of chemotherapy and GnRH agonist in ER-positive cancer is uncertain. Reduction of estrogen level caused by chemotherapy should also be taken into consideration. So, POF should not consider to endocrine responsive patients.
The functional recovery of ovaries is a clinical issue related to young breast cancer that should be taken into consideration with the increasing number of patients who desire to have a child after treatment.
Conclusion
We reported a case of ER-negative young breast cancer patient who achieved pregnancy and delivery after intensive adjuvant chemotherapy with POF. The baby is growing and developing without abnormality. It suggests fertility may be preserved by ovarian suppression with GnRH agonist during cytotoxic chemotherapy. It is important to accumulate reports of these cases because randomized control study is impossible for this issue.
Authors' Contribution
SK: Concept and design, preparation the draft manuscript.
TT: Supported in literature review and edited the manuscript.
NA: Edited the draft manuscript.
HK: Concept and design.
NO: Supported in literature review and edited the manuscript.
TI: Concept and design, edited the draft manuscript.
KH: Decision maker on management of the patient, edited the final manuscript.
Conflict of Interests
The all of authors have no conflicts of interest to disclose.
Ethical Considerations
Written consent of the patient was obtained for publication of this case report.
References
[1]. Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996; 14(5): 1718-1729. [Pubmed]
[2]. Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1997; 39: 1403-1409.[Pubmed]
[3]. Blumenfeld Z, Haim N. Prevention of gonadal damage during cytotoxic therapy. Ann Med. 1997; 29: 199-206.[Pubmed]
[4]. Recchia F, Saggio G, Amiconi G, Di Blasio A, Cesta A, Candeloro G, Rea S. Gonadotropin-releasing hormone analogues and added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006; 106: 514-523.[Pubmed]
[5]. Del Mastro L, Venturini M. Fertility preservation strategies for breast cancer patients. J Clin Oncol. 2006; 24: 4220-4221.[Pubmed]
[6]. Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Bio Reprod. 1995; 52: 365-372.[Pubmed]
[7]. Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ. 10th St. Gallen conference. Progress and promise : highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007; 18: 1133-1144.[Pubmed]
[8]. Walker RA, Lees E, Webb MB, Dearing SJ. Breast carcinomas occurring in young women (<35 years) are different. Br J Cancer. 1996; 74: 1796-1800.[Pubmed]
[9]. Chung M, Chang HR, Bland KI, Wanebo HJ. Young women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996; 77: 97-103.[Pubmed]
[10]. Kollias J, Elston CW, Ellis IO, Robertson JF, Blamey RW. Early-onset breast cancer--histopathological and prognostic considerations. Br J Cancer. 1997; 75: 1318-1323.[Pubmed]


