Original Article

Impact of Body Mass Index on Treatment Outcomes of Adjuvant Radiation Therapy in Saudi Females with Endometrial Carcinoma
2Mushabbab Al Asiri, 1Mutahir Ali Tunio, 3Abdul Rehman Alhaddab, 1Reham Mohammad, 3Yasser Bayoumi, 3Eyad Alsaeed, 4Abdullah Amro
- 1Assistant Consultant, Radiation Oncology, Comprehensive Cancer Center, King Fahad Medical City (KFMC), Riyadh 59046, Saudi Arabia
- 2Director and Chairman Radiation Oncology, Comprehensive Cancer Center, King Fahad Medical City (KFMC), Riyadh 59046, Saudi Arabia
- 3Consultant Radiation Oncology, Comprehensive Cancer Center, King Fahad Medical City (KFMC), Riyadh 59046, Saudi Arabia
- 4Chief Executive Officer, Consultant Radiation Oncology, Comprehensive Cancer Center, King Fahad Medical City (KFMC), Riyadh 59046, Saudi Arabia
- Submitted: October 18, 2012
- Accepted: November 22, 2012
- Published: December 18, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Background
Aim was to evaluate the impact of body mass index (BMI) on locoregional control (LRC), distant metastasis control (DMC), disease free survival (DFS) and overall survival (OS) in patients with endometrial carcinoma (EC) treated with adjuvant radiotherapy.
Materials and methods
Between June 2006 and July 2011, 66 patients with EC received adjuvant radiotherapy. Median age was 58.02 years (40-88). Mean BMI was 35.9 kg/m2 (23-72); BMI < 24 kg/m2 (normal weight) in 3 (4.5%), BMI 25-30 kg/m2 (overweight) in 19 (28.8%), BMI 31-40 kg/m2 (obese) in 20 (30.3%) and BMI > 40 kg/m2 (morbid obese) in 24 (36.4%).
Results
Median follow-up was 55 months (6-60). The Kaplan-Meier estimates of LRC, DMC, DFS and OS were 83.3%, 74%, 78.6% and 66.3% respectively. Patients with BMI > 30 kg/m2 showed inferior LRC (74.5%-80%) with p 0.003 and inferior OS (55%-61.4%) with p value 0.001. No influence of BMI on DMC and DFS was seen { hazard ratios of 0.97 (0.78-1.24) and 0.99 (0.81-1.26) respectively}. There was positive correlation of daily treatment setup errors with BMI > 30 kg/m2 (p 0.001). No correlation with found between BMI and radiation toxicity.
Conclusion
Patients with EC and high BMI had inferior LRC and OS. Emphasis shall be given on adjustment of setup errors during radiotherapy and on implementation of a national obesity prevention program.
Key words
Endometrial carcinoma, adjuvant radiotherapy, body mass index, treatment outcomes.
Introduction
Obesity and overweight (body mass index >30 kg.m2) are increasing among both sexes and across all age groups in Kingdom of Saudi Arabia with an overall prevalence of 44% in Saudi women [1]. Obesity is a known major risk factor for the development of endometrial carcinoma, which is the tenth most common and the second most common gynecologic malignancy in women in the Saudi Arabia [2]. Recent meta-analysis have shown that quantified risk of endometrial carcinoma is 1.59 (95% CIs: 1.50–1.68) per 5 kg/m2 increase in BMI [3].
Surgery is the primary treatment involving a total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymphadenectomy, and pelvic washings, with five year survival rates of 78% [4,5]. Randomized trials by Post-operative Radiation therapy in endometrial cancer (PORTEC) and Gynecological Oncology Group 99 (GOG-99) have shown significant reduction of the risk of pelvic and vaginal recurrence by adjuvant radiotherapy, although a survival benefit is not yet proven [6,7].
Elevated BMI is associated with increased mortality from other obesity-driven health problems, but retrospective data remained failed to show any impact of BMI on post treatment survival in patients with endometrial carcinoma [8,9]. Recently, results from MRC-ASTEC trial also have shown no influence of BMI on disease free survival and overall survival [10].
However, obese patients are at high risk of marginal failures because of poor target localization and potential setup errors while receiving radiotherapy, which may result in poor treatment outcome [11].
The purpose of this study was to evaluate the impact of body mass index (BMI) on locoregional control (LRC), distant metastasis control (DMC), disease free survival (DFS) and overall survival (OS), toxicity profile and magnitude of setup errors in patients with endometrial carcinoma (EC) treated with adjuvant radiotherapy.
Materials and Methods
After approval from Institutional Ethical Review Board (IRB) committee, between June 2007 and July 2011, sixty six consecutive patients with endometrial cancer treated with adjuvant radiotherapy in King Fahad Medical City comprised the study population.
All patients underwent surgery for endometrial cancer, including TAH, BSO and pelvic/para-aortic lymph node dissection for endometrial cancer. FIGO Stages ranged from IB to IIIC. Patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) Staging System. A detailed medical history, physical examination, hematology, serum chemistry, postoperative computed tomography (CT) of chest, abdomen and pelvis and central pathology review were performed to for accurate evaluation of extent of primary and nodal status and for assessment of any gross residual.
BMI calculation
Each patient was categorized according to BMI. Height and weight were measured at the time of accrual using institutional protocols and BMI was calculated using the formula of weight in kilograms divided by the square of the height in meters (kg/m2). BMI was then categorized into five groups as follows: underweight as BMI < 18.5 kg/m2; normal weight as BMI from 18.5 to 25 kg/m2; overweight as BMI from 25 to 30 kg/m2; obese as BMI from 31 to 40 kg/m2 and morbid obese as BMI above 40 kg/m2.
Treatment Protocol
All patients were simulated using Siemens Emotions 6 CT simulator. Contrast enhanced axial images of 5 mm slice thickness were obtained from the top of fourth lumbar (L4) vertebra to 5 cm below ischial tuberosities. After the acquisition of CT data, delineation of contouring of CTV [vaginal, cuff, parametria, external, internal iliac, presacral and common iliac lymph nodes], planning target volume (PTV) = CTV + 1 cm margin, and critical structures (urinary bladder, rectum, small bowel) was performed using Varian Eclipse Contouring software by two radiation Oncologists. After contouring treatment planning for conformal therapy (3DCRT) and intensity modulated radiotherapy (IMRT) was carried out by two medical physicists. Treatment plans were made using box field technique for 3DCRT or 5 to 7 dynamic beams for IMRT. The PTV was prescribed to 45-50.4 Gy in 25-28 fractions, 1.8 Gy per fraction, one fraction per day. Efforts were made to receive 45-50.4 Gy to 95% of PTV and to reduce hot spots less than 120%. During planning, total doses to the small bowel, rectum and bladder were constrained to < 40Gy, < 45Gy and 45 Gy respectively (Fig.1a).
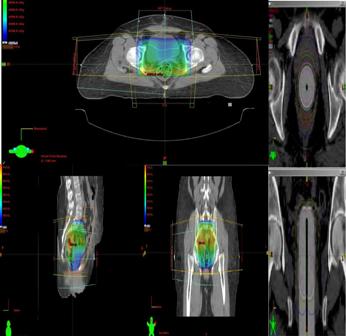
Fig.1: (a)External beam radiation therapy (3DCRT) planning for endometrial carcinoma in axial, coronal and saggital section and (b) intravaginal brachytherapy.
Weekly treatment verification
All patients were treated on Varian linear accelerator (LA, Clinac®) with on-board imaging (OBI). All patients were initially setup on LA using their anterior and lateral tattoos for the alignment. Subsequently, orthogonal (lateral and anterior/posterior) kilovoltage images were acquired and sent to the OBI workstation, where they were registered to the digitally reconstructed radiographs (DRR) from their treatment planning CT scan. A two dimensional match was performed using bony anatomy by a senior therapist and online translational shifts in 1 or > 1 millimeters (lateral, longitudinal, and vertical) before starting treatment. Weekly images were obtained unless there was an issue with the imager or unexpected shift. These shifts were verified off-line by a radiation oncologist. Median 15 (7-25) images per patient were obtained. The mean absolute daily shifts in the vertical, longitudinal, and lateral directions were calculated.
After completion of pelvic external beam irradiation, high dose rate (HDR) intravaginal brachytherapy (IVBT) was given. Total IVBT dose was 15 Gy delivered in 3 sessions (each session three days apart). The reference point for dose prescription was 0.5 cm from surface of vaginal applicators (Fig.1b).
Adjuvant chemotherapy
Adjuvant chemotherapy was given to patients IB grade3, IIA, IIB, IIIA, IIIB, IIIC, serous papillary or clear cell histology patients before starting radiation therapy. Adjuvant chemotherapy consisted of four to six cycles of paclitaxel (175mg/m2) and carboplatin (350mg/m2) every 3 weeks based on Lupe K, et al Protocol [12].
Toxicity and Response evaluation
During radiation therapy, patients were evaluated every week for weight, performance status, hematology/chemistry and side effects. The National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0, were used to score acute radiation toxicity (≤90 days from start of radiation therapy). The Radiation Therapy Oncology Group (RTOG) Late Radiation Morbidity Scoring Criteria were used to score radiation toxicity persisting beyond 90 days from the completion of radiotherapy.
After completion of therapy, periodic follow ups were carried out every 3 months for first two years and every 6 months subsequent 3rd to 5th year with physical and per vaginal examination, pap smear and CT chest, abdomen and pelvis.
Statistical analysis
The primary endpoints were LRC, DMC and toxicity profile. Secondary endpoints were DFS, OS and dosimetric parameters including the magnitude of setup errors and shifts during the radiation therapy. The times to last follow up evaluation, appearance of local and distant relapse and death were calculated from date of starting treatment. DFS was defined as the duration between the entry date and the date of documented disease reappearance, death from cancer and/or last follow-up (censored). OS was defined as the duration between the entry date and the date of patient death or last follow-up (censored). Probabilities of LRC and DMC, DFS and OS were determined with the Kaplan-Meier method. The comparisons for various endpoints were performed using log rank test and Cox regression analysis. Treatment set up errors and correlation between BMI was carried out using logistic regression. Univariate and multivariate analyses were also performed for different prognostic factors. All statistical analyses were performed using the computer program SPSS version 16.0.
Result
Median follow up was 55 months (range: 6-60 months). Patients' characteristics according to BMI subgroups are shown in (Table 1). Majority of study cohort was with cormorbids (36 patients, 54.5%) and median BMI was 35.9 kg/m2 (range: 23-72). Majority of study cohort was with BMI above 30 kg/m2 (44 patients, 66.7%). Grade 1 and grade 3 was predominant histological pattern (48 patients, 76.8%). Most common stage was, IB in 29 patients (43.9%) followed by IIIC in 12 patients (18.2%) and node positive (N1) disease in 5 patients (7.6%). Twenty four patients (36.4%) received additional chemotherapy.
Table 1: Patients characteristics according to body mass index category.
| Variables |
BMI < 25 kg/m2
N=3 (4.5%) |
BMI 25-30 kg/m2
N=19 (28.8%) |
BMI 31-40 kg/m2
N=20 (30.3%) |
BMI > 40 kg/m2
N=24 (36.4%) |
P value |
Comorbids
(DM/HTN) |
2 (3.05%) |
8 (12.1%) |
11 (16.7%) |
15 (22.5%) |
0.02 |
| Stage |
|
|
|
|
|
| IA |
1 (1.5%) |
- |
1 (1.5%) |
2 (3.05%) |
|
| IB |
- |
5 (7.6%) |
6 (9.1%) |
18 (27.3%) |
|
| IIA |
- |
1 (1.5%) |
3 (4.5%) |
2 (3.05%) |
|
| IIB |
- |
1 (1.5%) |
3 (3.5%) |
2 (3.05%) |
0.01 |
| IIIA |
1 (1.5%) |
5 (7.6%) |
2 (3.05%) |
- |
|
| IIIB |
1 (1.5%) |
- |
- |
- |
|
| IIIC |
- |
7 (10.6%) |
5 (7.6%) |
- |
|
| Cell Type |
|
|
|
|
|
| Endometroid |
2 (3.05%) |
19 (28.8%) |
20 (30.3%) |
20 (30.3%) |
0.05 |
| Serous papillary |
- |
- |
- |
4 (6.1%) |
|
| Clear cell |
1 (1.5%) |
- |
- |
4 (6.1%) |
|
| Pathological grade |
|
|
|
|
|
| G1 |
1 (1.5%) |
4 (6.1%) |
8 (12.1%) |
11 (16.7%) |
0.9 |
| G2 |
2 (3.05%) |
6 (9.1%) |
6 (9.1%) |
4 (6.1%) |
|
| G3 |
- |
9 (13.6%) |
6 (9.1%) |
9 (13.6%) |
|
| LVI |
|
|
|
|
|
| Yes |
- |
9 (13.6%) |
8 (12.1%) |
1 (1.5%) |
0.05 |
| No |
3 (4.5%) |
10 (15.2%) |
12 (18.2%) |
23 (34.9%) |
|
| ER/ PR Receptors |
|
|
|
|
|
| Positive |
- |
- |
1 (1.5%) |
10 (15.2%) |
0.03 |
| Negative |
3 (4.5%) |
19 (28.8%) |
19 (28.8%) |
14 (21.2%) |
|
| LN |
|
|
|
|
|
| Positive |
1 (1.5%) |
2 (3.05%) |
1 (1.5%) |
1 (1.5%) |
0.08 |
| Negative |
2 (3.05%) |
17 (25.8%) |
19 (28.8%) |
23 (34.9%) |
|
| Squamous Metaplasia |
|
|
|
|
|
| Yes |
- |
- |
- |
6 (9.1%) |
0.02 |
| No |
3 (4.5%) |
19 (28.8%) |
20 (30.3%) |
18 (27.3%) |
|
Adjuvant
chemotherapy |
|
|
|
|
|
| Yes |
2 (3.05%) |
8 (12.1%) |
5 (7.6%) |
4 (6.1%) |
|
| No |
- |
11 (16.7%) |
15 (22.8%) |
20 (30.3%) |
0.9 |
| Dose RT |
|
|
|
|
|
| 45 Gy EBRT + IVBT |
3 (4.5%) |
17 (25.8%) |
15 (22.8%) |
20 (30.3%) |
|
15 Gy
50.4 Gy EBRT+ IVBT
15 Gy |
- |
2 (3.05%) |
5 (22.8%) |
4 (6.1%) |
0.8 |
Median time between surgery and radiotherapy was 7.1 weeks (range: 5-24). The median dose to PTV was 47.5 Gy (range: 45-50.4) and IVBT dose was 15 Gy at 0.5 cm from surface of applicators and mean radiotherapy duration was 6.5 weeks (range: 6- 8).
Toxicity profile
Treatment was generally well tolerated by all patients with grade 1 and 2 acute side effects (Table 2). No grade 3 and 4 side effects or hospitalization or treatment related death was seen. No correlation was noticed between BMI and radiation toxicity profile. Late toxicity was seen only in one patient who presented with sub-acute intestinal obstruction which was managed conservatively.
Table 2: Incidence of grade 2 and 3 acute and late toxicities
|
Grade 1, 2 n (%) |
Grade 3, 4 n (%) |
| Toxicity |
Acute |
Late |
Acute |
Late |
| Hematological |
|
|
|
|
| Anemia |
0 |
0 |
0 |
0 |
| Neutropenia |
0 |
0 |
0 |
0 |
| Thrombocytopenia |
0 |
0 |
0 |
0 |
| Skin |
5 (7.6%) |
1 (1.5%) |
0 |
1 (1.5%) |
| Small Bowel |
4 (6.1%) |
0 |
0 |
1 (1.5%) |
Proctitis
(diarrhea, rectal pain ) |
3 (4.5%) |
1 (1.5%) |
0 |
0 |
| Nausea/vomiting |
4 (6.1%) |
0 |
0 |
0 |
Vagintis
(adhesion/fibrosis) |
3 (4.5%) |
1 (1.5%) |
0 |
0 |
| Cystitis |
2 (3.1%) |
0 |
0 |
0 |
Locoregional control, distant control and overall survival rates
The Kaplan-Meier estimates of locoregional control, distant metastasis control, disease free survival and overall survival were 83.3%, 74%, 78.6% and 66.3% respectively (Fig.2).
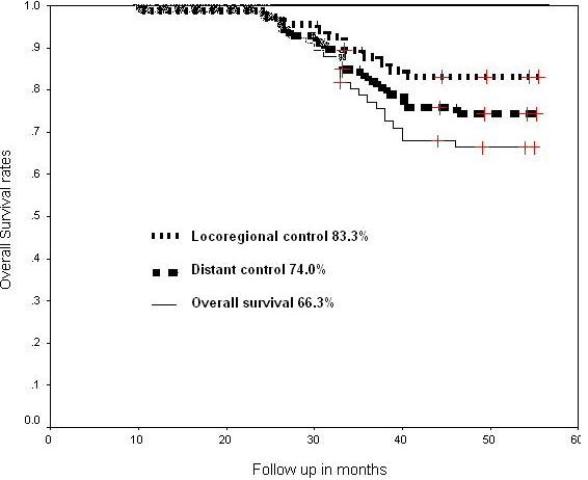
Fig.2. Cumulative locoregional control, distant control and overall survival at 55 months of follow up.
Eleven patients developed locoregional al recurrences (in-field). Two patients had vaginal recurrences (3.02%) and eight patients had pelvic nodal recurrence (12.2%). Of whom ten locoregional recurrences were seen in patients with BMI >30 kg.m2 (p value 0.003) as shown (Fig.3). For these patients salvage surgery was done in 5 patients (7.6%) and salvage chemotherapy for 4 patients (6.1%). Two patients (3.05%) refused for any further treatment (Table 3).
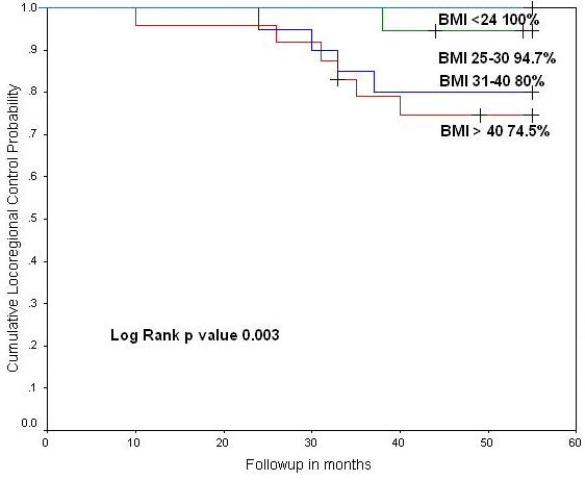
Fig.3: Cumulative (a) locoregional control and (b) hazard ratio according to body mass index category at 55 months of follow up.
Distant metastases were seen 16 patients (24.4%) (Table 3). Of whom 7 patients (10.6%) had simultaneous locoregional failure. First event was seen at 10 months of completion of adjuvant radiotherapy. Fourteen patients (21.2%) received salvage chemotherapy. No correlation was seen between BMI and distant metastasis control.
Table 3: Locoregional Recurrences according to body mass index and treatment.
| Recurrence |
BMI |
Time of first
recurrence |
Salvage Treatment |
Status
At 55 months |
Disease Free survival
(%)
at 55 months |
| Locoregional |
|
|
|
|
|
| Vaginal |
1 (1.5%) 31-40 |
23 months |
Surgery |
1 dead with disease |
|
| 2 (3.02%) |
1 (1.50%) > 40 |
10 months |
Surgery |
|
50% |
|
|
|
Surgery |
1 disease free alive |
|
| Pelvic |
1 (1.5%) 25-30 |
38 months |
Refused 2 (3.05%) |
6 dead with disease |
33.4% |
| 9 (12.2%) |
3 (4.5%) 31-40 |
23 months |
Chemotherapy 4 (6.1%) |
3 disease free alive |
|
|
5 (7.6%) > 40 |
10 months |
|
|
|
| Distant |
|
|
|
|
|
| Lungs |
4 (6.1%) <24 |
10 months |
Chemotherapy 7 (10.6) |
8 dead with disease |
|
| 8 (12.2%) |
2 (3.02%) 31-40 |
|
Refused 1 (1.5%) |
|
|
|
2 (3.02%) > 40 |
|
|
1 disease free alive |
|
| Bones |
1 (1.5%) 25-30 |
31 months |
Chemotherapy 3 (4.5%) |
1 alive with disease |
|
| 4 (6.1%) |
2 (3.02%) 31-40 |
|
Refused 1 (1.5%) |
2 dead with disease |
12.5% |
|
1 (1.5%) > 40 |
|
|
|
|
| Para-aortic nodes |
1 (1.5%) <24 |
10 months |
Chemotherapy 3 (4.5%) |
1 disease free alive |
|
| 3 (4.5%) |
1 (1.5%) 25-30 |
|
|
2 dead with disease |
|
|
1 (1.5%) > 40 |
|
|
|
|
| Brain |
|
36 months |
Cranial irradiation |
1 Dead with disease |
|
| 1 (1.5%) |
1 (1.5%) <24 |
|
Chemotherapy (1.5%) |
|
|
At 55 months of follow-up, 21 patients (31.85%) were dead. Of whom 5 patients (23.8%) died of non endometrial cancer related causes. Patients with BMI > 30 had more mortality from non- endometrial cancer related causes (p 0.001).
Further univariate and multivariate analyses were carried out (Table 4). It was found that BMI, FIGO stage and LVI were significant prognostic factors for locoregional failure (p 0.02, 0.05 and 0.03 respectively). For distant failure, FIGO was significant prognostic factors (p 0.02). For overall survival BMI and FIGO stage were found significant prognostic factors (p 0.01 and 0.02 respectively).
Table 4: Univariate and multivariate analyses of variables on locoregional control, distant control and overall survival.
| Variable |
Locoregional Control P value OR (95% CI) |
Distant Metastasis Control P value OR (95% CI) |
Overall Survival P value OR (95%CI) |
| Age (< 50 vs. > 50 years) |
0.33 0.93 (0.90-1.50) |
0.66 1.10 (0.89-2.00) |
0.71 0.50 (0.10-2.41) |
| Cormorbids (Yes vs. No) |
0.98 0.88 (0.67-0.97) |
0.901.80 (0.79-2.10) |
1.00 1.80 (0.79-2.10) |
| FIGO stage (IIB) |
0.05 3.34 (2.52-10.34) |
0.02 3.65 (1.81-9.65) |
0.01 3.85 (1.91-10.35) |
| N stage (N0 vs. N1) |
0.77 1.10 (0.89-2.00) |
0.661.10 (0.89-2.00) |
0.56 1.21 (1.10-2.10) |
| BMI kg/m2 (>30 vs. <30) |
0.02 3.45 (1.61-9.45) |
0.56 1.10 (0.89-2.00) |
0.02 3.65 (1.81-9.65) |
| Cell type ( Endometroid vs. non Endometroid) |
0.48 1.21 (1.10-2.10) |
0.60 1.10 (0.89-2.00) |
0.77 1.21 (1.10-2.10) |
| Grade (<G2 vs.G3) |
0.23 0.97 (0.95-1.13) |
0.90 0.88 (0.67-0.97) |
0.23 0.97 (0.95-1.13) |
| LVI ( no vs. yes) |
0.03 2.21 (1.45-7.85) |
0.90 0.88 (0.67-0.97) |
0.60 1.10 (0.89-2.00) |
| Adjuvant chemotherapy (Yes vs. No) |
0.75 0.50 (0.10-2.41) |
0.44 0.93 (0.90-1.50) |
0.70 0.50 (0.10-2.41 |
| EBRT dose (45 Gy vs. 50.4) |
0.45 0.78 (0.23-2.38) |
0.450.78 (0.23-2.38) |
0.56 1.10 (0.89-2.00) |
| Squamous metaplasia (Yes vs. No) |
0.85 1.80 (0.79-2.10) |
0.400.78 (0.23-2.38) |
0.33 0.93 (0.90-1.50) |
During radiotherapy set up error frequency
Overall the mean shifts of offline portal imaging review from digitally reconstructed radiographs (DRR) ranged from 2.5 to 5 mm in the vertical direction, 2.5 to 5 mm in the longitudinal direction and 3 to 6.6 mm lateral direction. On linear regression, there was positive correlation between mean absolute vertical, longitudinal, and lateral shifts and BMI (p 0.02, p 0.01, and p 0.001, respectively) (Table 5).
Table 5: Radiotherapy setup errors (systemic and random) of the weekly shifts according to body mass index (BMI)
| Shifts (millimeters) |
BMI < 25 kg/m2 |
BMI 25-30 kg/m2 |
BMI 31-40 kg/m2 |
BMI > 40 kg/m2 |
P value |
| Vertical |
2.5 (1.8-2.7) |
2.7 (2.1-3.1) |
3.0 (2.8-4.1) |
5.0 (3.5-5.5) |
0.02 |
| Longitudinal |
2.5 (2.1-3.1) |
2.6 (2.3-3.5) |
4.0 (3.0-4.5) |
5.0 (4.0-5.8) |
0.01 |
| Lateral |
2.5 (2.2-3) |
3.0 (2.7-3.8) |
4.0 (3.8-5.0) |
6.6 (4.2-7.4) |
0.001 |
Discussion
The results of our study showed that elevated BMI > 30 Kg/ m2 is associated with reduced locoregional control (p value 0.003) and overall survival (p 0.001) in patients with endometrial carcinoma receiving adjuvant radiotherapy, yet there was no effect of BMI on distant recurrences and disease free survival. However, the influence of body mass index on locoregional control and overall has remained controversial so far. The prospective trial by Gynecologic Oncology Group (GOG) speculated on 380 patients with early endometrial carcinoma, that elevated BMI was associated with increased overall mortality (Hazard ratio = 2.76, 95% CIs: 1.20–6.33 for BMI 40 kg/m2), but no effect on locoregional recurrences [13]. Temkin et al, found in 442 patients with early endometrial carcinoma that elevated BMI may be associated with a better survival, but after adjusting the potential confounders, this effect was not seen [14]. Similar beneficial effects of elevated BMI were reported by Modesitt et al and Mauland et al [15,16]. However these beneficial effects of BMI were criticized for selection bias. Contrary, Everett et al and Martra et al found no influence of BMI on cancer-specific survival in 396 and 766 surgically-treated endometrial cancer patients, respectively [8,9]. Recently, results of MRC-ASTEC trial based on BMI available for 1070 patients with early endometrial carcinoma showed no impact of elevated BMI on locoregional control and overall survival [10]. However, this trial was criticized for its recruitment bias and study population was early stage endometrial cancer. Our study, 21 patients (31.8%) were Stage III; sufficient to reduce confounder bias when analyzing the impact of BMI on treatment outcomes. Further in our study, the patients with elevated BMI > 30 kg/m2 were mainly with good prognostic features, i.e., early stage disease and less lymphovascular invasion and ER and PR receptor positivity. Higher locoregional recurrences in our patients with BMI > 30 kg/m2 in the presence of early stage and favorable histological features might be explained increased setup errors and larger margins and shifts requirements with reduced therapeutic ratio in these patients. Several studies has shown significant correlation between systematic errors in the lateral and longitudinal direction and BMI, with errors highest among > 30 kg/m2 group making the treatment less reproducible in these patients which potentially result sub-optimal dose the target and critical structures organs at risk [11,17,18]. These results warrants the consideration of adjusting the setup errors and margins according to the BMI of individual patient with or without help of image guided radiation therapy (IGRT) daily basis [19]. However no correlation was seen between elevated BMI and radiation therapy induced acute or delayed toxicity profile. No treatment related death was observed in study population.
All vaginal recurrences were salvaged with surgical treatment in our study, but this group showed reduced DFS (at 55 months, 50%) as compared to patient without vaginal recurrences. Overall high distant metastases in our study population can be explained by inclusion of advance stage disease patients in the study and the possible explanation for decreased overall survival in our study is that hypertension and diabetes were present in 39.2% patients with elevated BMI > 30 kg/m2 with increased mortality from other obesity-driven health issues.
Potential strengths to support our data were, [1] to determine BMI, height and weight were measured rather than self-reported, BMI was available for all patients, [2] reduced confounder bias by analyzing all data for clinico-pathological covariates, [3] majority of study cohort was with BMI > 30 kg/m2 and all patients were treated with standardized allocated treatment. Low sample size was only limitation of our study.
Conclusion
Our study showed less aggressive stage and histological features in obese patients (BMI > 30 kg/m2) with endometrial carcinoma, still were at higher risk of locoregional recurrences; likely because of suboptimal dose distribution secondary to systematic errors and shifts in the lateral and longitudinal direction during the radiation therapy. These results warrant frequent adjustments of the setup errors and margins according to the BMI of individual patient with or without help of IGRT. Reduced overall survival in patients with BMI > 30 s kg/m2 secondary to obesity driven health issues warrants weight reduction strategies among the patients and implementation of national obesity prevention program.
Authors' Contribution
MAA, MAT: Design of study.
AAH, YB: Data collection.
EAS, AAH, YB: Data analysis.
MH, MAT: Data writing.
MAA, MAT, AA: Final Approval.
Conflict of Interests
Authors do not have any potential conflict of interest to declare. No grants or funds received for conducting and publishing this study.
List of abbreviations
BMI: Body mass index.
LRC: Locoregional control.
DMC: Distant metastasis control.
DFS: Disease free survival.
OS: Overall survival.
EC: Endometrial carcinoma .
PORTEC: Post-operative Radiation therapy in endometrial cancer.
GOG: Gynecologic Oncology Group.
CT: Computed tomography.
CTV: Clinical target volume.
PTV: planning target volume.
3DCRT: three dimensional conformal radiation therapies.
IMRT: Intensity modulated radiation therapy.
IGRT: Image guided radiation therapy.
OBI: On board imaging.
DRR: Digitally reconstructed radiographs.
IVBT: intravaginal brachytherapy.
References
[1]. Al-Nozha MM, Al-Mazrou YY, Al-Maatouq MA, Arafah MR, Khalil MZ, Khan NB, Al-Marzouki K, Abdullah MA, Al-khadra AH, Al-Harthi SS, Al-Shahid MS, Al-Mobeireek A, Nouh MS. Obesity in Saudi Arabia. Saudi Med Journal 2005;26:824-9. [Pubmed].
[2]. Cancer incidence report Saudi Arabia 1999-2000.
[3]. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579–91. [Pubmed].
[4]. Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, Santaquilani M; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EUROCARE-4 study. Lancet Oncol 2007;8:773–83. [Pubmed].
[5]. Obermair A, Manolitsas TP, Leung Y, Hammond IG, McCartney AJ. Total laparoscopic hysterectomy for endometrial cancer: patterns of recurrence and survival. Gynecol Oncol 2004;92:789-93. [Pubmed].
[6]. Creutzberg CL, Nout RA, Lybeert ML, Warlam-Rodenhuis CC, Jobsen JJ, Mens JW, Lutgens LC, Pras E, van de Poll-Franse LV, van Putten WL; PORTEC Study Group. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int Radiat Oncol Biol Phys 2011;81:e631-8. [Pubmed].
[7]. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG; Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol 2004;92:744-51. [Pubmed].
[8]. Everett E, Tamimi H, Greer B, Swisher E, Paley P, Mandel L, Goff B. The effect of body mass index on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol 2003;90:150–7. [Pubmed].
[9]. Martra F, Kunos C, Gibbons H, Zola P, Galletto L, DeBernardo R, von Gruenigen V. Adjuvant treatment and survival in obese women with endometrial cancer: an international collaborative study. Am J Obstet Gynecol 2008;198:89 [e1–8]. [Pubmed].
[10]. Crosbie EJ, Roberts C, Qian W, Swart AM, Kitchener HC, Renehan AG. Body mass index does not influence post-treatment survival in early stage endometrial cancer: Results from MRC ASTEC trial. Eur J Cancer 2012;48:853-64. [Pubmed].
[11]. Wong JR, Gao Z, Merrick S, Wilson P, Uematsu M, Woo K, Cheng CW. Potential for higher treatment failure in obese patients: Correlation of elevated body mass index and increased daily prostate deviations from the radiation beam isocenters in an analysis of 1,465 computed tomographic images. Int J Radiat Oncol Biol Phys 2009;75:49-55. [Pubmed].
[12]. Lupe K, Kwon J, Dsouza D, Gawlik C, Stitt L, Whiston F, Nascu P, Wong E, Carey MS. Adjuvant paclitaxel and carboplatin chemotherapy with involved field radiation in advanced endometrial cancer: a sequential approach. Int J Radiat Oncol Biol Phys 2007;67:110-6. [Pubmed].
[13]. von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma : a Gynecologic Oncology Group study. Cancer. 2006;107:2786-91. [Pubmed].
[14]. Temkin SM, Pezzullo JC, Hellmann M, Lee YC, Abulafia O. Is body mass index an independent risk factor of survival among patients with endometrial cancer? Am J Clin Oncol 2007;30:8–14. [Pubmed].
[15]. Trovik J, Mauland KK, Werner HM, Wik E, Helland H, Salvesen HB. Improved survival related to changes in endometrial cancer treatment, a 30-year population based perspective. Gynecol Oncol 2012;125:381-7. [Pubmed].
[16]. Modesitt SC, Tian C, Kryscio R, Thigpen JT, Randall ME, Gallion HH, Fleming GF; Gynecologic Oncology Group. Impact of body mass index on treatment outcomes in endometrial cancer patients receiving doxorubicin and cisplatin: a Gynecologic Oncology Group study. Gynecol Oncol 2007;105:59-65. [Pubmed].
[17]. Johansen J, Bertelsen A, Hansen CR, Westberg J, Hansen O, Brink C. Set-up errors in patients undergoing image guided radiation treatment. Relationship to body mass index and weight loss. Acta Oncol 2008;47:1454-8. [Pubmed].
[18]. Bortfeld T, van Herk M, Jiang SB. When should systematic patient positioning errors in radiotherapy be corrected? Phys Med Biol 2002;47:N297eN302. [Pubmed].
[19]. Lin LL, Hertan L, Rengan R, Kevin Teo BK. Effect of Body Mass Index on Magnitude of Setup Errors in Patients Treated with Adjuvant Radiotherapy for Endometrial Cancer with Daily Image Guidance. Int J Radiat Oncol Biol Phys. 2011; 83: 670-5. [Pubmed].


