Original Article

Clinico-pathological response of neoadjuvant chemotherapy in advance ocular carcinoma
1Rajendra P Maurya, 1Prashant Bhushan, 1Virendra P Singh, 1Mahendra K Singh, 2Manoj Pandey, 3Udai P Shahi, 4Mohan Kumar
- 1Department of Ophthalmology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- 2Department of Surgical Oncology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- 3Department of Radiotherapy & Radiation Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- 4Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- Submitted: February 10, 2011;
- Accepted February 24, 2012
- Published: March 5, 2012
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objective:
To determine the feasibility, toxicity and clinico-pathological response of anterior chemotherapy in patients of advanced ocular malignancies.
Settings:
Departments of Ophthalmology, Surgical Oncology and Radiotherapy and Radiation Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, U.P., India.
Materials and Methods:
Twenty six patients of ocular malignancies between 20 to 65 years of age were treated with chemotherapy (CT) (I.V. cis-platinum, methotrexate, bleomycin) in 16 cases and only injection methotrexate in 10 cases prior to radiotherapy and/or surgery. Before starting chemotherapy all patients were evaluated by history, clinical examination, complete hematological, radiological and pathological investigations. Drug toxicity, clinical and histological changes were observed after completion of treatment.
Results:
Out of 26 cases, 21 were primary malignant tumors arising from eye and its adnexa and 5 were secondary malignant tumors arising from paranasal sinuses. Majority of cases had squamous cell carcinoma 16/26 (61.54%) followed by basal cell carcinoma 5/26 (19.23%) and Meibomian gland carcinoma 5/26 (19.23%). Overall response of CT was seen in 19/26 (73.08%) cases, while in 5/26 (19.23%) cases there was no response and rest 2/26 (7.69%) had progressive disease. The maximum response 12/26 (46.15%) was observed in patients treated by combination chemotherapy containing cis-platinum. There was mild to moderate drug toxicity, commonest were gastrointestinal (69.23%) and hematological (69.23%).
Conclusion:
Chemotherapy is better adjuvant to surgery and radiotherapy in advanced ocular malignancies.
Introduction
Ocular malignancies are rather complex group of conditions which comprise not only primary tumors arising from ocular adnexa and orbit itself, but also malignant tumors extending from neighboring structures particularly adjoining paranasal sinuses and cranial fossa.
Many forms of treatment have been described for ocular tumors. Surgery is the conventional method, associated with high rate of cure and excellent results. Sometimes surgery is not possible due to poor general condition of patients or due to extensive disfigurement with functional loss patients refuse to have surgery [1]. Other methods of treatment for ocular tumors including radiotherapy, chemotherapy, phototherapy and cryotherapy have been described [1,2,3] The use of chemotherapy in the management of ocular malignancies have rapidly progressed since last two to three decades when the earliest reports were published [1-6] The advantages of anterior chemotherapy prior to radiotherapy and/or surgery are (i) reduction in tumor size may allow for less extensive surgery or less intensive radiation (ii) may destroy micrometastasis (iii) if radiotherapy is given before chemotherapy it could interfere with tumor blood supply hence resulting in reduced response of chemotherapy.
Material and Methods
Twenty six patients who presented with advanced ocular malignancies but without distant metastasis and without any prior therapy for the existing malignancy and in sufficiently good general condition to tolerate the chemotherapy were selected. Prior to therapy thorough assessment of all patients including complete history, physical examination, ocular and ENT examination were done. Neck was examined for lymph node metastasis. Routine hematological tests, urine examination and chest X-ray were done in all patients. Other radiological investigations like computerized tomography and USG were performed wherever clinically indicated. Blood urea nitrogen level upto 20mg/100ml, serum creatinine upto 1.5 mg/100ml and white blood cell count of 4000 mm3 or more and platelet count of 100000/mm3 or more were used as a laboratory screening parameters. Clinical photograph and histopathological examination were done before and after complete cycle of chemotherapy in all cases.
The treatment schedules adopted were as follows : (i) 10 patients were treated by single drug regimens – which included injection methotrexate 50 mg I.V. weekly for six week (ii) 16 patients were treated by two cycle of multi-drug regimens including PMB (cis-platinum, methotraxate and bleomycine). These drugs were given in following doses and schedule – cis-platinum 30 mg I.V on day 1, 2, 3, 4, 5 monthly for 2 months Methotrexate 25 mg I.V. on day 1, 8 monthly for 2 months and Bleomycin 15 mg I.V. on day 1, 8 monthly for 2 months. Before delivering chemotherapy, patients were properly hydrated with I.V. injection of 5% dextrose 1 liter in 2 to 4 hours, injection 5% dextrose saline 1 liter in 4 to 6 hours and injection normal saline 400 ml in next one hour. Injection cis-platinum 30 mg was given in last 150 ml of normal saline over one hour. Injection dextromethasone 8 mg, and metaclopropamide 25 mg was administered 10-15 minutes before giving cis-platinum. Injection mannitol 100 ml was given 10 minutes after completion of chemotherapy.
Toxicity was monitored by blood count, blood urea and serum creatinine during treatment and before starting the next cycle. Reduction in dose of chemotherapy was done according to creatinine level/creatinine clearance. Radiotherapy and/or surgery were done after 2-3 weeks gap. Patients were kept in regular follow-up. Finally drug toxicity, clinical and histopathological response of chemotherapy were evaluated. Clinical response was assessed as per the criteria of the “WHO hand book”[7].
Response was evaluated as follows
Complete response (CR): Disappearance of all known disease, Partial response (PR): Fifty percent or more decrease in tumor size; No response (NR): less than 50% decrease or less than 25% increase in tumor size; Progressive disease (PD): Twenty five percent or more increase in tumor size or appearance of new lesions.
Table 1 : Patient Characteristics
| Characteristics |
|
No |
% |
| Gender |
|
|
|
|
Male |
18 |
69.23 |
|
Female |
8 |
30.77 |
| Localization |
|
|
|
|
Lid |
18 |
69.23 |
|
Conjunctiva |
3 |
11.54 |
|
Paranasal Sinuses |
5 |
19.23 |
Types of
Malignancy |
|
|
|
|
Squamous cell
carcinoma |
16 |
61.54 |
|
Primary |
11 |
42.31 |
|
Secondary |
5 |
19.23 |
|
Basal Cell
carcinoma |
5 |
19.23 |
|
Meibomian gland
carcinoma |
5 |
19.23 |
| Differentiation |
|
|
|
|
Well |
7 |
26.92 |
|
Moderate |
9 |
34.62 |
|
Poorly |
10 |
38.46 |
Results
Out of 26 patients, 18 (69.23%) were men and 8 (30.77%) were women. Age ranged from 20 to 65 years, majority (73.07%) of patients were between 45 to 70 years. A total of 21/26 (80.77%) had primary malignant tumors arising from eye and its adnexa and 5/26 (19.23%) had secondary arising from paranasal sinuses (Figure 3). Majority of cases had squamous cell carcinoma (61.54%) (Figure 1,2), followed by basal cell carcinoma (19.23%) and Meibomian gland carcinoma (19.23%). The histopathological grading was well differentiated type in 26.92% cases, moderately differentiated in 34.62% cases and poorly differentiated type in 38.46% cases (Table 1,Figure 4). Chemotherapy was completed 92.31% patients according to protocol but was modified (cis-platinum was stopped during second cycle) for two patients because of patients clinical condition or side effects. 65.38% patients underwent for radiotherapy and in 34.62% cases surgery was performed 2 to 3 weeks after chemotherapy. There was no mortality related to chemotherapy. Results were evaluated clinically in all cases and histologically in 61.5% cases. Clinical response to chemotherapy was as follow: 38.46% patients had complete response and 34.62% showed partial response, with overall response (CR+PR) in 73.08% cases. No response was seen in 5/26 (19.23%) cases and progression in 2/26 (7.69%) (Table 2). Overall response to CT in well differentiated carcinoma was 57.1%, in moderately differentiated carcinoma it was 66.6% while in poorly differentiated carcinoma it was 90% (Table 3). histological changes in those patients who had complete response were flattening of mucosa, keratinization and spongiosis of overlying epithelium along with mononuclear infiltration and occasional scattered cancer cells (Figure 5).
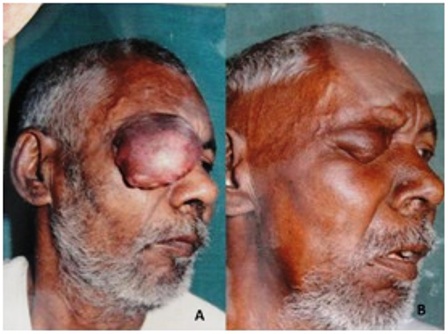
Figure 1: (a) A pre-treatment photograph of patient of squamous cell carcinoma right upper eyelid; (b) A post treatment photograph of same patient. Note the marked reduction in size of the tumor and complete disappearance of pre-auricular lymph node.
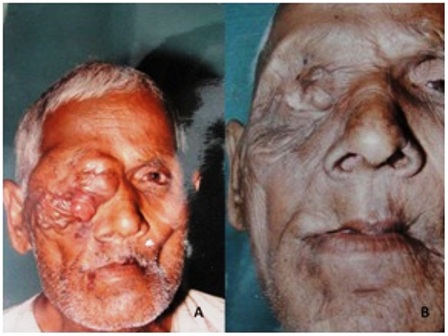
Figure 2: (a) Clinical photograph of patient showing recurrence (after surgery) of squamous cell carcinoma of right upper eyelid; (b) A post treatment clinical photograph of same patient. Note the marked reduction in size of the tumor

Figure 3: (a) Clinical photograph of young patient of carcinoma left ethmoidal sinus, showing proptosis; (b) A post treatment clinical photograph of same patient. Note complete disappearance of proptosis; (c) Clinical photograph of same patient showing post chemotherapy alopecia.
The toxicities associated with chemotherapy is given in table-4. None of the patients had serious or fatal side effects related to the chemotherapy. Commonest toxicity were mild to moderate gastrointestinal (69.2%) and hematological (69.2%) toxicity. Most patients had nausea who received injection cis-platinum infusion which was well controlled with antiemetics. 15.4% patients had alopecia and 7.69% patients had renal toxicity for which cis-platinum was stopped in next cycle.
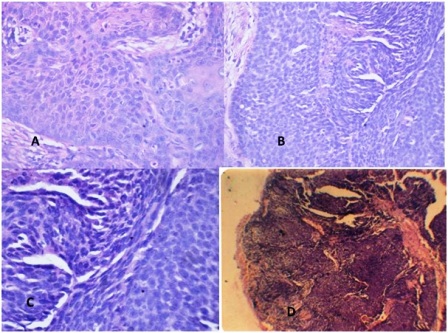
Figure 4 Photomicrograph of (a) Well differentiated squamous cell carcinoma grade I (Hematoxylin and Eosin, X100) (b) Moderately differentiated squamous cell carcinoma grade II (Hematoxylin and Eosin, X125) (c) Poorly differentiated squamous cell carcinoma (Hematoxylin and Eosin, X100) (d) Moderately differentiated sebaceous gland carcinoma grade II, Showing lobules of malignant cells with sebaceous differentiation (Hematoxylin and Eosin, X100)
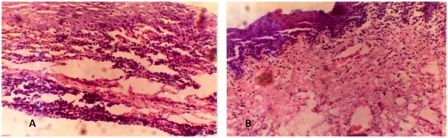
Figure 5: (a) Photomicrograph of post chemotherapy biopsy showing dense lymphatic infiltration. There is a slight thickening of epithelium with spongiosis; (b) Photomicrograph of post chemotherapy biopsy showing cleavage in epithelium with mostly round cell infiltration and fibrovascular proliferation in deeper layer. Total clearing of cancer cells.
Discussion
For successful long term cure of ocular malignancies, as of any part of the body, total eradication of tumor is needed by treating it aggressively in its initial therapy. The commonest cause of tumor recurrence is inadequate therapy with part of tumor being left behind. The combination of various treatment modalities such as chemotherapy and or radiotherapy with surgical excision of ocular tumor minimizes the recurrence rate.
The objective of our study was to find out the role and response of anterior chemotherapy (both single and combination drug regimen) given prior to surgery or radiotherapy in advanced ocular malignancies. In our study, 8.4% patients were treated by single drug regimen (methotrexate) and 61.5% were treated by combination chemotherapy (PMB, cis-platinum, bleomycin and methotrexate). Cis-platinum is the only heavy metal compound used as chemotherapeutic agent. Cis-platinum directly attack on DNA, cause change in DNA conformation and inhibition of DNA synthesis [8]. In our study, overall response (CR+PR) rate was 73.0% and there was no response (NR) in 19.2% while in 7.6% cases disease progressed. Luxenberg and Guthrie (1985) use cis-platinum chemotherapy for basal cell carcinoma and squamous cell carcinoma of eyelids and periocular skin with following response rate, CR in 62.5% and PR in 37.5% patients [1]. While Michael Morley et al., (1990) reported PR in 100% of cases of basal cell carcinoma of lid treated by cis-platinum [5].
Table 2 : Clinical response to chemotherapy
| Response |
Single Drug Regimen
(MTX) |
Multi Drug Regimen
(PMB) |
Total |
|
No |
% |
No |
% |
|
Complete Response
(CR) |
3/10 |
30 |
7/16 |
43.75 |
10/26
38.45% |
Partial Response
(PR) |
4/10 |
40 |
5/16 |
31.25 |
9/26 34.62% |
No Response (NR) +
Progressive disease
(PD) |
(2+1)=
3/10 |
(20+10)=
30 |
(3+1)=
4/16 |
(18.75+
6.25)=
25 |
(5+2)/26
(19.23
+7.69)=
26.92% |
Table 3 : Response to chemotherapy according to histopathological grading
| Histo-pathological Grading |
Single Drug Regimen (MTX) |
Multi Drug Regimen (PMB) |
|
CR |
PR |
NR |
CR+PR |
CR |
PR |
NR |
CR+
PR |
Well Differentiated
Number
% |
-- |
1/3
33.33 |
2/3
66.67 |
1/3
33.33 |
2/4
50 |
1/4
25 |
1/4
25 |
3/4
75 |
Moderately
Number
% |
1/3
33.33 |
1/3
33.33 |
1/3
33.33 |
2/3
66.67 |
2/6
33.33 |
2/6
33.33 |
2/6
33.33 |
4/6
66.67 |
| Poorly Differentiated Number % |
2/2/4
50 |
2/4
50 |
-- |
4/4/4
100 |
3/6
50 |
2/6
33.33 |
1/6
16.67 |
5/6
83.33 |
In our study, the complete response rate was slightly higher (38.45%) in multi drug regimen group as compared to single drug regimen (30%) but overall (CR+PR) rate was more or less equal in both single and multidrug regimen groups (70% vs. 73.0%). Chemotherapy has the best effect on poorly differentiated (90%) and moderately differentiated (66.6%) carcinoma as compared to well differentiated carcinoma (57.1%) (table 3).
Chemotherapy is not always safe. Many side effects associated with chemotherapy have been reported. The common non-specific side effects were gastrointestinal, hematological and alopecia. The significant side effects seen with intravenous cis-platinum chemotherapy were nephrotoxicity, ototoxicity and myelosuppression [5,9-11]. Reports describing the ocular toxicities of cis-platinum chemotherapy are: retrobulbar neuritis and papilledema [12], cortical blindness and transient visual field defects [13,14] and abnormalities of visually evoked potentials and electroretinogram [11,13,14]. None of our patient had severe toxicity related to chemotherapy. In our series commonest toxicities were gastrointestinal and hematological those were mild, generally tolerable and self limiting.
Table 4: Incidence of toxicity
| Types of toxicity |
Number |
% |
| Hematological |
18 |
69.23 |
| - Leokopenia |
10 |
38.46 |
| - Thrombocytopenia |
3 |
11.54 |
| - Anaemia |
5 |
19.23 |
| Gastrointestinal |
18 |
69.23 |
| Nausea & Vomiting |
18 |
69.23 |
| - Diarrhea |
8 |
30.77 |
| - Stomatitis |
5 |
19.23 |
| Alopecia |
4 |
15.38 |
| Renal toxicity |
2 |
7.69 |
Conclusions
As anterior chemotherapy reduces the tumor bulk it is better than adjuvant therapy after surgery and radiotherapy in advanced ocular malignancies. Multidrug regimens have better response rate than single drug regimen but are more costly and toxic. Response is better in poorly and undifferentiated carcinoma than well differentiated ones.
Authors’ contributions
RPM: Performed literature search, conducted the study and prepared the manuscript.
PB: Conducted the literature search and designed the study under supervision
VPS: collection, interpretation and analysis of clinical data
MKS: designed the study, analysis and interpretation of data and review and revision of final manuscript.
MP: Interpretation of data and editing of final manuscript for scientific content.
UPS: carried out the clinical trial, read and edited the final manuscript, evaluation of clinical response.
MK: collection interpretation of pathological data, contribution to pathological part of manuscript.
All authors read and approved the final manuscript
RPM is the guaranteer of the study.
Funding Support:
None
Conflict of Interests
The authors declare that there is no conflict of interests.
References
[1]. Luxenberg MN, Guthrie TH. Chemotherapy of eyelid and periorbital tumors. Trans Am Ophthalmol Soc 1985; 83 :162-180.
[2]. Luxenberg MN, Guthrie TH. Chemotherapy of basal cell and squamous cell carcinoma of the eyelids and periorbital tissues. Ophthalmology 1986; 93: 504-510.
[3]. Rabinson JK. Use of combination chemotherapy and radiation therapy in the management of advanced basal cell carcinoma of the head and Neck. J Am Acad Dermatol 1987; 17: 770-774.
[4]. Fitzpatrick PJ, Thompson GA, Easterbrook WM, Gallie BL, Payne DG. Basal and squamous cell carcinoma of eyelid and their treatment by radiotherapy. Int J Radiat Oncol Biol Phys 1984; 10: 449-454.
[5]. Morley M, Finger PT, Perlin M, Weiselberg LR, DeBlasio DS. Cis-platinum chemotherapy for ocular basal cell carcinoma. Br J Ophthalmol 1991; 75: 407-410.
[6]. Beard C. Observations on the treatment of basal cell carcinoma of the eyelids. The Wendell L. Huges Lecture. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 1975; 79: 664-670.
[7]. World Health Organization. WHO handbook on reporting results of Cancer treatment. WHO publication no 48, 1979, Geneva.
[8]. Rosenberg B. Fundamental studies with cis-platinum. Cancer 1985; 55: 2303-2316.
[9]. Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cis-platin toxicity. Am J Kidney Dis 1986; 8: 368-379.
[10]. Piel IJ, Meyer D, Perlia CP, Wolfe VI. Effects of cis-diamminedichloroplatinum (NSC-119875) on hearing function in man. Cancer chemother Rep 1974; 58: 871-875.
[11]. Griffin JD, Garnick MB. Eye toxicity of cancer chemotherapy; a review of the literature. Cancer 1981; 48: 1539-1549.
[12]. Ostrow S, Hahn D, Wiernik PH, Richards RD. Ophthalmic toxicity after cis-dischlorodiammineplatinum (II) therapy. Cancer Treat Rep 1978; 62: 1591-1594.
[13]. Berman IJ, Mann MP. Seizures and transient cortical blindness associated with cis-platinum (II) diamminedichloride (PPD) therapy in a thirty-year old man. Cancer 1980; 45: 764-766.
[14]. Pippitt CH Jr, Muss HB, Homesley HD, Jobson VW. Cis-platinum associated cortical blindness. Gynecol Oncol 1981; 12 (2 Pt 1): 253-255.


