Original Article

Intraoperative Ultrasound in Intracranial Space Occupying Lesions
Deepak Patil, Vivek Sharma, V Divye Prakash Tiwari
- *Department of Neurosurgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- Friday, November 01, 2013
- Sunday, November 03, 2013
- Thursday, November 14, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective
The aim was to assess the role of intraoperative ultrasound in intracranial space occupying lesion.
Methods
We have performed intraoperative ultrasound in 192 patients
of cranial space occupying lesions admitted at our university hospital.
Intraoperative ultrasound was performed by SonoSite, 180 plus hand carried
ultrasound system with c11/7-4 Mhz curved array transducer probe. A preoperative
Computed tomography or Magnetic resonance was performed in all patients.
Preoperative and postoperative neurological function was assessed.
Observations
Intraoperative ultrasound examination is an excellent device
in localizing the small intracranial space occupying lesion. It also helps in
planning the durotomy and extending the craniotomy size if required. It helps in
identifying the shortest and safest site to approach the lesion, and helps in
preventing the damage to eloquent areas. In cases of cystic tumor with small
solid component, it helps in guiding the cyst puncture and exact localization of
the solid component. In cases of large intracranial space occupying lesions,
post excision intraoperative ultrasound helps in delineating the completeness of
resection. Residual tumor if seen in ultrasound was excised.
Conclusion:
Intraoperative ultrasound is portable and does not require
any specialized setup. It is cost effective and provides real time images. It
can be repeated as and when required during operation with minimum scanning
time, and ensures patient and operator safety.
Keywords
Intraoperative ultrasound,glioma, meningioma,intracranial tumour, brain neoplasm
Introduction
Preoperative images in Magnetic Resonance Imaging (MRI) or Computerized
Tomography (CT) data is commonly used for guidance during surgery.
It is important to localize the tumour on the surface of brain so that unnecessary neural damage can be avoided. Thus per-operative ultrasound helps to a great extent but many different factors like drainage of cerebrospinal fluid, excision of tumor mass and position of the patient intraoperatively affect the tissue alignment and may cause problem called brain shift
[1,5].
Intraoperative imaging modalities like intra operative
ultrasound, intraoperative CT, and intraoperative MRI have been introduced to
know real time images reflecting the true patient’s anatomy. Intraoperative
ultrasound imaging of the brain is being performed usually by transducers with 5
or 7.5 MHz. The 5 MHz probe retains the ability to visualize up to the depth of
15 cm while 7.5 MHz probe is used for more superficial application [6].
Ultrasound waves do not propagate efficiently in air the space therefore saline
is being used as acoustic medium between the surface of the transducer and the
brain [7].
Patients and Methods
This study included 192 cases of cranial space occupying
lesions who were admitted in the Department of Neurosurgery, Institute of
Medical Sciences, Banaras Hindu University between 2011 to 2013. The mean age of
the patients was 48 (+/- 28) years and the male to female ratio was 51 to 45.
The detailed clinical history including name, age, sex, social status, history
related to etiological factors, onset and duration of disease was taken.
Thorough physical examination was performed. The Chief Complaints like headache,
nausea, vomiting, seizures, dysphasia, loss of consciousness, cerebellar
involvement-nystagmus, intentional tremor, unsteadiness of gait, cranial nerve
involvement, limb weakness, behavioral changes were noted. Past history like
previous operation and radiation, type of intervention and outcome of
intervention were noted. Preoperative investigations like computerized
tomography and magnetic resonance imaging were done. The Ultrasonography was
performed at the beginning and end of operation.
Intraoperative Details
Intraoperative ultrasound (IOUS) with SonoSite, 180 plus with
c11/7-4 Mhz curved array transducer probe was done in all cases. The ultrasound
probe, covered with an aseptic sheath, was placed directly on the surface of the
duramater exposed by craniotomy, and saline was used as acoustic coupling agent.
The probe was manipulated on the examining surface to obtain coronal and
sagittal views of the lesion through the window formed by the removal of bone.
Intraoperative ultrasonographic findings like tumor location, cortical sulci
above the tumor, depth of the tumor, relationship with the surrounding
anatomical structures, and extent of resection at the end of surgery were noted.
Results
In our study majority of the patients (52.8%) were in the age
group of 41-60 years and the average age was 52.8 year. There were 57 patients
in the age group of 21-40 years, 37 patients in the age group of 0-20 years and
26 patients in the 61 years and above age group with mean age of 31.1, 9.6 and
66 respectively. The younger patient was 6 year old while the oldest was 73 year
old. The male patients outnumbered the female counterpart (53.13% versus
46.87%). Supratentorial lesions accounted for 136 cases (70.83%) whereas
infratentorial cases were 56 (29.17%). The supratentorial lesions were
distributed in the frontal, temporal, parietal and occipital lobe in 52
(38.24%), 36(26.47%), 28 (20.59%) and 20(14.70%) respectively.
Intraoperative frozen section and postoperative
histopathological findings confirmed the diagnosis. Meningioma accounted for the
highest number of supratentorial cases (54,39.70%). High grade gliomas
(Glioblastoma and Anaplastic Astrocytomas) accounted for maximum cases of
gliomas (31,22.80%) whereas low grade gliomas were found in 20 cases (14.70%).
Six out of 10 cases of abscess were tubercular (4.41%) while 4 (2.94%) were
pyogenic. There were 10 cases of Metastasis (7.35%). The Hydatid cyst, Arachnoid
cyst and Neurocysticercosis were reported in 4 (2.94%), 4 (2.94%) and 2 (1.47%)
cases respectively. Among infratentorial ICSOLs, Acoustic neuroma/schwannoma
accounted for maximum cases (11, 19.64). Pilocytic astrocytoma & Meningioma
were reported in 10 cases each (17.86%). Medulloblastoma (6,10.71%), Ependymoma
(6,10.72%), Metastasis(3,5.36%), Arachnoid cyst (3,5.36%), Neurocysticercosis
(1,1.78%), Hemangioblastoma (2,3.57%) and Abscess (4,7.14%) were the remaining
cases.
Among supratentorial (n=136), 95 cases underwent Gross tumor
resection (69.85%), while subtotal resection and biopsy in 26 (19.12%) and 15
(11.03%) cases respectively. Among infratentorial group (n=56), 50 cases
underwent gross tumor resection (89.29%). Subtotal resection and diagnostic
biopsy was performed in 4 (7.14%) and 2 cases (3.57%). (table 1)
| Supratentorial (n=136) |
No. of patients |
Percentage |
| Gross tumor resection |
95 |
69.85 |
| Subtotal resection |
26 |
19.12 |
| Biopsy |
15 |
11.03 |
| Infratentorial (n=56) |
| Gross tumor resection |
50 |
89.29 |
| Subtotal resection |
04 |
7.14 |
| Biopsy |
02 |
3.57 |
Among 41 supratentorial meningiomas on IOUS, all had clear delineation of border (100%), 38 were hyperechoic (92.68%) Figure 1 , 39 tumors had homogeneous interior (95.12%), 20 tumors had hypoechoic surrounding edema (48.78%).
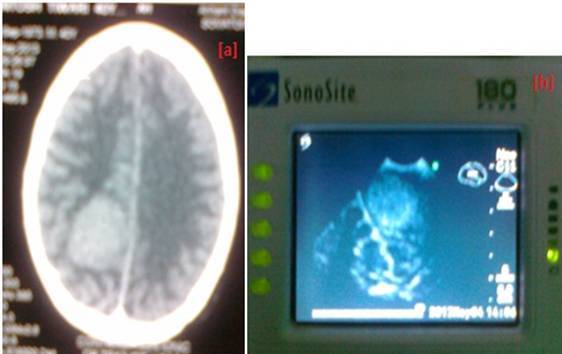
Figure 1. Right parieto-occipital meningioma (a).preoperative CT Scan, (b).IOUS image showing tumor in relation to falx
Edema was absent in 15 cases (36.58%) and edema not defined in 6 cases (14.63%).
Table 2.
| Meningioma |
No. of patients |
Percentage |
| Clear delineation of border |
41 |
100 |
| Hyperechoic tumor |
38 |
92.68 |
| Homogeneous interior |
39 |
95.12 |
| Hypoechoic surrounding edema |
20 |
48.78 |
| No edema |
15 |
36.58 |
| edema not defined |
06 |
14.63 |
Among 51 supratentorial Glioma on IOUS, 23 tumor were hyperechoic (45.10%) Figure 2, 19 had mixed echogenicity (37.25%) while 9 were hypoechoic (17.65%).
Thirty two tumors had well defined border (62.74%) and 46 tumors had perifocal edema (90.20%).
Table 3
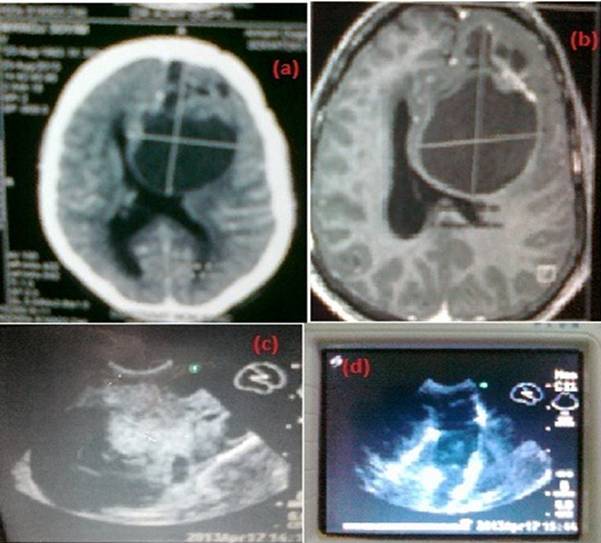
Fig 2. A 28 years old male with left frontal glioma (a large cyst with small solid component) (a). preoperative CT scan, (b). MRI Axial, (c).Intraoperative ultrasound, (d).Intraoperative ultrasound postexcision
Immature abscesses had indistinct margin, irregular shape and
variable echogenicity. Mature abscess had distinct margin, regular shape with
hypoechoic nature. Cerebral neurocysticercosis had distinct margin, irregular
shape with hyperechoic scolex in cyst. Arachnoid cyst was uniformly hypoechoic.
Metastases were mostly homogeneous iso or hyperechoic well delineated lesions Figure 3.
Hydatid cyst was uniformly hypoechoic.
| Glioma |
No. of patients |
Percentage |
| Tumour hyperechoic |
23 |
45.10 |
| Mixed echogenicity |
19 |
37.25 |
| Tumour hypoechoic |
09 |
17.65 |
| Well defined border |
32 |
62.74 |
| Perifocal edema |
46 |
90.20 |
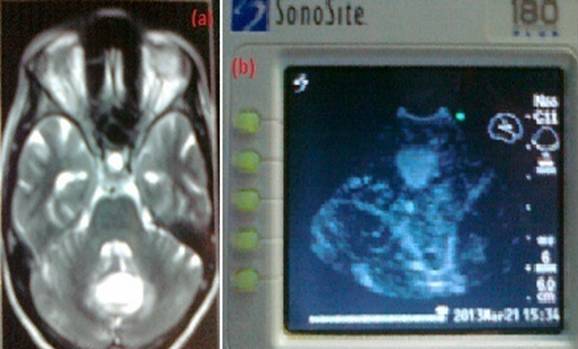
Fig.3.Vermian metastatic adenocarcinoma (a).T2 MRI image, (b). Intraoperative Ultrasound
Lesions of the Posterior Fossa
Meningioma, Gliomas, Metastases, cysts had similar appearance
as of their supratentorial counterpart. Cerebellopontine angle tumors were
clearly visualized. IOUS of the posterior fossa was useful as a guide for tumor
biopsies, and for the detection of small subcortical lesions. Hemangioblastoma
in the cerebellum appeared as a hypoechoic cyst with hyperechoic small mural
nodule on IOUS.
Discussion
Solheim et al, (2010) published a series of 156 malignant gliomas: 142 (91%) were resected while 14 (9%) were undergone biopsies. [8] They reported gross total resection (GTR) in 37% of all high-grade glioma resection. Chacko et al, (2003) evaluated thirty-five patients with parenchymal brain lesions including 11 low-grade and 22 high-grade tumours and 2 inflammatory granulomata. [9]. They found all tumours irrespective of histology to be hyperechoic on IOUS. In 71.4% of cases, IOUS was useful in defining their margins, however in the remaining cases the margins were illdefined. Wang et al, (2011) evaluated 52 patients with small subcortical lesion. [10].In
our study we found that IOUS examination is excellent in localizing small
intracranial space occupying lesions. In 30 cases of gliomas we also performed
tumor localization with neuronavigation. Before durotomy neuronavigation was
fairly accurate in localizing the gliomas matching the IOUS localization.
However after durotomy and resection of tumors with alteration in tissue
dynamics with cerebrospinal fluid loss, tumor tissue loss, neuronavigation
proved inaccurate in 19 cases because of brain shift. We found that IOUS helps
in planning the durotomy and if needed extending the craniotomy. It helps in
identifying the shortest and safest approach to the lesion and helps in
preventing damage to vital areas. In cases of cystic tumors with small solid
component, IOUS helps in guiding the cyst puncture and exact localization of the
solid component. In cases of large intracranial space occupying lesions post
excision intraoperative ultrasound helps in delineating the completeness of
resection. Residual tumor if left was excised. Neurosurgeons who have used real
time 2D ultrtasound for different procedures, have found 3 D ultrasound
cumbersome because the probe comes on the way of instruments. Special attention
must be paid to positioning the surgical instrument accurately in the real time
2D US scan plane to see and guide the surgical instrument in the image. In
addition, extra space is needed in the craniotomy for simultaneous placement of
both the US probe and the instrument. The main drawbacks of intraoperative CT
are the huge amount of radiation exposure to the patient during surgery,
escalation of radiation exposure with repeated imaging, additional radiologic
staff dedicated for intraoperative purposes and the exorbitant cost related to
build an intraoperative brain suite that will not be available at many
institutions especially in developing countries. The main difficulties with the
use of intraoperative MRI are requirement of non-ferromagnetic instruments,
MR-compatible devices including operating microscope, additional technician &
dedicated neuroradiologist experienced in intraoperative MR images as
intraoperative MR images are different from pre- or postoperative ones because
of the air-brain-interface and changes due to surgical manipulation e.g. blood
clots. An additional 60 to 90 minutes of anesthesia time is needed. The transfer
to intraoperative MRI suite is cumbersome and repeated imaging at different
stages of operation if needed is not feasible. IOUS is a cheaper solution and it
is safe for both the patient and operator.
Conclusion
IOUS is portable, does not require any specialized setup,
provides real time images, cost effective, and can be repeated as and when
required during the procedure with minimum scanning time. It also ensures
patient and operator safety. IOUS can be performed without increasing operation
time significantly. It might even shorten it because it increases the surgeons’
feeling of safety.
Authors' Contribution
DP: Collection of data, and preparation of the manuscript.
VS: Concept and design, preparation of the manuscript.
DPT: Literature review
and preparation of the manuscript.
All authors have read and approved the
final manuscript for publication.
Conflict of Interests
The authors declare that there are no conflict of interests.
Ethical Considerations
This is a retrospective review and is exempted from Ethical
Committee review.
Funding
None declared
Acknowledgement
None
References
[1].Koivukangas J, Louhisalmi Y, Alakuijala J, Oikarinen J .Neuronavigaton-guided cerebral biopsy. Acta Neurochir Suppl:1993b; 58: 71–74.[pubmed]
[2].Koivukangas J, Louhisalmi Y, Alakuijala J & Oikarinen J. Ultrasound-controlled neuronavigator-guided brain surgery. J Neurosurg :1993a;79: 36–42.[pubmed].
[3].Hirschberg H, and Unsgaard G. Incorporation of ultrasonic imaging in an optically coupled frameless stereotactic system. Acta Neurochir Suppl Wien.1997:68;75-80.[pubmed]
[4].Koivukangas J, Louhisalmi Y, Alakuijala J, and Oikarinen J. Ultrasound-controlled neuronavigator-guided brain surgery. J Neurosurg:1993;79:36-42.[pubmed]
[5].Trobaugh JW, Richard WD, Smith KR, and Bucholz RD. Frameless stereotactic ultrasonography: Method and applications. Comput Med Imaging Graph.1994:18:235-246.[pubmed].
[6].Sutcliffe JF (1991) Review article: The value of intraoperative ultrasound in neurosurgery. British Journal of Neurosurgery.1991: 5; 169–178.[pubmed].
[7].Selbekk T, Jakola A S, Johansen T F, Lindseth F, Reinertsen I, Unsgård G.(2013)Ultrasound imaging in neurosurgery: approaches to minimize surgically induced image artefacts for improved resection control, Acta Neurochir.2013:013;1647-7.[pubmed]
[8].Solheim O, Selbekk T, Jakola A,Unsgård G. Ultrasound-guided operations in unselected high-grade gliomas—overall results, impact of image quality and patient selection. Acta Neurochir.2010: 152;1873–1886.[pubmed]
[9].Chacko A G,. Kumar N K S Chacko G, Athyal R, Rajshekhar V(2003) Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours – a comparative study with computed tomography and histopathology Acta Neurochir.2003: 145; 743–748.[pubmed]
[10].Wang J DuanY Liu X, Wang Y, Gao G , Qin H,Wang L Application of Intraoperative Ultrasonography for Guiding Microneurosurgical Resection of Small Subcortical Lesions Korean J Radiol.2011:12(5);541-546.[pubmed]


