Original Article

A prospective study examining the onset and progression of acute toxicity and patient-reported side effects during altered fractionation radiotherapy with concomitant boost for oropharyngeal cancer
1Bena Cartmill, 2Petrea Cornwell, 3Elizabeth Ward, 4Wendy Davidson, 5Sandro Porceddu
- 1Medical and Health Research Fellow, Centre for Functioning and Health Research, Queensland Health, Speech Pathologist - Advanced (Oncology), Princess Alexandra Hospital, P.O. Box 6053, Buranda, 4102 Australia
- 2Senior Research Fellow, Metro North Health Service District, Queensland Health, Australia, and Griffith Health Institute, Griffith University, Mt Gravatt Campus, 176 Messines Ridge Road, Mt Gravatt QLD 4111
- 3Centre for Functioning & Health Research, Queensland Health, and The University of Queensland, Division of Speech Pathology, P.O. Box 6053, Buranda, 4102 Australia
- 4Department of Dietetics, Princess Alexandra Hospital, Queensland Health,Ipswich Rd, Woolloongabba Q 4102
- 5The University of Queensland, School of Medicine, and Department of Radiation Oncology, Princess Alexandra Hospital, Queensland Health, Ipswich Rd, Woolloongabba Q 4102
- Submitted: March 18, 2013
- Accepted: May 24, 2013
- Published: June 05, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Acute grade 3 and 4 toxicity is commonly reported in trials examining altered fractionation radiotherapy (AFRT), due to its impact on treatment tolerance, the potential for consequential late effects, and mortality. Less well described is the mild-moderate acute toxicity and its impact on function. This study aims to examine acute toxicity and patient-reported side effects, and how they impact on function during AFRT.
Study Design
A prospective cohort design study.
Materials and Methods
Thirteen patients with T1-T3 oropharyngeal SCC were assessed weekly during, and at four weeks post-AFRT. Acute toxicity was graded using the CTCAE, and patients attended speech pathology/dietetic reviews where they reported functional barriers. Swallowing and weight measures were recorded.
Results
Most participants experienced peak grade 2 toxicity for all CTCAE components, except laryngeal edema. Grade 3 mucositis and dysphagia was noted in 31% and 23% respectively. Peak toxicity occurred in week 5 of treatment; however barriers to oral intake occurred from week 1. Modified diet with supplementation was required for 92% by week 3. Participants lost 5 kg during treatment. By four weeks post-treatment acute toxicity was resolving, with ongoing diet modification and weight loss.
Conclusion
Mild-moderate acute toxicity impacted on functional swallowing and weight from weeks 1-2, with increasing severity by week 3, before patients commenced their twice daily “concomitant boost” treatments. As acute toxicity resolved, the impact on oral intake and weight continued. Future studies should record toxicity and barriers to oral intake routinely until its resolution, rather than at arbitrary time points post-treatment.
Key words
acute toxicity, oropharyngeal cancer, altered fractionation radiotherapy, swallowing, nutrition.
Introduction
The benefits with regards to survival and locoregional control in treating locally advanced head and neck squamous cell carcinoma with altered fractionation radiotherapy (AFRT) have been well-recognized [1]. One type of AFRT uses a concomitant boost (AFRT-CB) regimen which involves the delivery of a smaller second daily dose in the final days/weeks of treatment, and has been found to have significantly worse acute toxicity when compared with conventionally fractionated radiotherapy [2]. This fractionation protocol is being examined further in a prospective phase II TROG clinical trial (registered number: A0031029V). Patient-reported side effects and their impact on functional outcomes during AFRT treatment have not been described extensively.
Most reports of toxicity following radiotherapy treatment have focused primarily on grade 3 and 4 adverse events due to the impact such severe toxicity has on treatment completion, compliance and mortality. Severe acute toxicity has been associated with treatment breaks, weight loss, alternative feeding and hospitalization [3-6]. Studies within the last decade however have largely reported a pattern of reduced incidence of grade 3 and 4 acute toxicity following concomitant boost protocols, with literature finding that between 20-32% developed grade 3 or 4 dysphasia toxicity and 35-47% developing grade 3 or 4 mucositis [2, 7-9]. However, in comparison, there has been less consistent reporting of mild-moderate acute toxicity following concomitant boost radiotherapy. Where data has been reported, it has revealed that the large majority of patients developed grade 1 or 2 acute toxicity for erythema (68-85%) [2, 7, 9], xerostomia (67-100%) [2, 7-9], mucositis (40-80%) [2, 7-9], and dysphasia (42-68%) [2, 7, 9], although no study has reported the clinical progression of when toxicity begins and at what stage it reaches maximal severity. Also less discussed in the literature to date is the functional impact of mild-moderate acute toxicity on functional swallowing and nutritional status. The challenges of toxicity reporting have been noted previously and include a lack of documented standards and poor consensus regarding reporting [10]. This limits the current understanding of the onset, progression, duration, and recovery from acute toxicity.
Information regarding the pattern of acute toxicity, as well as patient-reported side effects of treatment is critical to assist service planning in relation to the nature and extent of speech pathology and dietetics support required by this population during and early after treatment. This paper aimed to: 1) prospectively examine the full range of early toxicity weekly during treatment to determine the onset and progression of acute toxicity; 2) determine the patterns of presentation and the peak severity and incidence of acute toxicity; and 3) describe the patient-reported barriers to oral intake and how they impact on functional swallowing and weight in patients with T1-T3 locally advanced head and neck squamous cell carcinoma of the oropharynx treated with AFRT-CB.
Material and Methods
Participants
Patients with a diagnosis of T1, T2, or T3N0 SCC of the oropharynx (tonsil, base of tongue, pharyngeal wall or supraglottis [within 1cm of the oropharynx]) who presented to the Princess Alexandra Hospital Multidisciplinary Head and Neck Clinic in Brisbane, Australia between November 2006 and August 2009, and were treated with AFRT-CB, were eligible for recruitment. HPV p16 status was not reliably reported during this time at our institution, and thus is unknown in the investigated cohort. Ineligible patients included those with previous diagnosis of head and neck cancer, neurological or neurodegenerative condition that may have affected oral intake. All patients received their treatment at the Metro South Radiation Oncology Service in Brisbane, Australia. This research was approved by the Human Research and Ethics Committees at the Princess Alexandra Hospital, Australia, and the University of Queensland, which conform to the provisions under the Declaration of Helsinki. All participants provided written consent prior to involvement in the study.
AFRT-CB was the recommended treatment for 17 patients during the study period, all of whom were eligible for recruitment. Two patients declined to participate, leaving a cohort of 15 participants who consented to involvement. Of these participants, complete data on 13 participants was available; one participant’s data set was incomplete as a result of death unrelated to cancer and another excluded due to inadequate attendance for weekly progress sessions.
Participants were predominantly male (11 male, 2 female) with a mean age of 67 years (SD = 9.02; range = 53-82). Demographic and clinical characteristics are reported in Table 1. The majority of participants had tonsillar (n = 8, 62%) and supraglottic (within 1cm of the oropharynx; n = 3, 23%) primary disease. Fifty-four percent of the participants had T2 tumours and the majority of patients (69%) had no nodal disease (N0). The majority of participants had stage III (39%) or stage II disease (31%). More than half (54%) of the participants were ex-smokers, 23% current smokers, and 85% reported being current alcohol drinkers. Prior to treatment mean weight of the cohort was 83kg (SD = 23.63).
Table 1: Demographics of AFRT-CB cohort at presentation
Participant
Number |
Age (years) |
Sex* |
TNM Classification† |
Stage |
Smoking |
Alcohol |
Weight
(kgs)
|
| 1 |
82 |
M |
T1N0 left pharyngeal wall |
I |
Ex |
Current |
70.3 |
| 2 |
63 |
M |
T2N0 supraglottic |
II |
Current |
Current |
77.8 |
| 3 |
79 |
M |
T3N0 BOT |
III |
Ex |
Current |
74.5 |
| 4 |
72 |
M |
T2N0 left tonsil |
II |
Never |
Current |
75.2 |
| 5 |
69 |
F |
T2N2b left tonsil |
IV |
Ex |
N/A |
73 |
| 6 |
73 |
M |
T2N0 left tonsil |
II |
Ex |
Ex |
61 |
| 7 |
70 |
M |
T1N0 left tonsil |
I |
Ex |
Current |
106.7 |
| 8 |
69 |
M |
T2N1 right tonsil |
III |
Ex |
Current |
65.8 |
| 9 |
69 |
M |
T3N0 right supraglottic |
III |
Current |
Current |
81 |
| 10 |
59 |
M |
T2N0 right supraglottic |
II |
Ex |
Current |
81 |
| 11 |
58 |
F |
T3N0 right tonsil |
III |
Current |
Current |
59.5 |
| 12 |
53 |
M |
T2N1 right tonsil |
III |
Never |
Current |
142 |
| 13 |
54 |
M |
T1N2a right tonsil |
IV |
Never |
Current |
113 |
* M = male, F = female. †T = T stage, N = N stage.
Planned Treatment
Patients received a total radiotherapy dose of 66Gy in 35 fractions over 5 weeks. All patients were treated with 3D conformal radiotherapy using curative intent AFRT-CB. Elective sites were treated to 50Gy in 2Gy/day over 5 weeks. Known sites of disease received a concomitant boost schedule to a total of 66Gy over 5 weeks with an afternoon boost dose (minimum of 6 hours apart) of 1.6Gy/day in weeks 4 and 5.
Outcome Measures
Participants were examined prospectively each week during treatment (week 1 to 5) with a further follow-up assessment at four weeks following completion of treatment. Table 2 outlines the assessment schedule and outcome measures used by each professional. At each time point, the radiation oncologist scored treatment toxicity using the CTCAE v.3 [11]. Toxicity was recorded in each participant’s medical chart during the review appointment with their treating radiation oncologist.
In addition, at each time point participants underwent combined speech pathology and dietetic review assessments. Rather than speech pathology and dietetic involvement occurring reactively in response to swallowing difficulties or weight loss, routine intervention was provided on a weekly basis during treatment. In the speech pathology assessment, participants were interviewed and asked to report the side effects that they perceived were barriers to their oral intake. They also underwent a clinical swallow examination to determine the tolerance and safety of diet and fluid consistencies, examination of the oral cavity, and recommendations regarding oral cares and strategies to manage barriers to oral intake (Table 2). The dietitian completed assessment of weight, diet history, prescription and monitoring of nutritional supplements nd/or alternative feeding and recommendations to manage barriers impacting on oral intake (Table 2). Symptom management was provided in conjunction with the multidisciplinary team.
Table 2: Assessment schedule for radiation oncologist, speech pathologist, and dietitian during treatment and at four weeks post-AFRT-CB
| Outcome Measure |
Time point |
|
Pre |
Wk§ 1 |
Wk2 |
Wk3 |
Wk 4 |
Wk5 |
4wks
post |
Radiation Oncology Assessment:
CTCAE v.3*
Symptom management |
|
|
|
|
|
|
|
|
• |
• |
• |
• |
• |
• |
|
• |
• |
• |
• |
• |
• |
Speech Pathology Assessment:
CSE†
Oral cavity examination
Recommendations‡ re:
Dry mouth/thick secretions
Odynophagia
Trismus
Oral ulceration
Oral candidiasis |
|
|
|
|
|
|
|
| • |
• |
• |
• |
• |
• |
• |
|
• |
• |
• |
• |
• |
|
|
• |
• |
• |
• |
• |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dietitian Assessment:
Weight
Diet history
Supplements/alternative feeding
Recommendations‡ re:
Anorexia
Dysgeusia
Nausea/ vomiting
Diarrhoea
Constipation
Dysosmia
Early satiety
Timely access to food |
|
|
|
|
|
|
|
| • |
• |
• |
• |
• |
• |
• |
| • |
• |
• |
• |
• |
• |
|
| • |
• |
• |
• |
• |
• |
|
|
• |
• |
• |
• |
• |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*Common Toxicity Criteria for Adverse Events version 3.0. †Clinical swallow examination. ‡Recommendations and symptom management provided in conjunction with multidisciplinary team. §Wk/s = week/weeks.
Statistical analysis
All data was recorded on a set “Swallowing and Nutrition Proforma” to ensure consistent reporting and then entered into an Excel spreadsheet. Data were entered into Stata v.10 for Mac. Analysis was by intention-to-treat; therefore, if a patient missed one review appointment, but not consecutive appointments, the last observations obtained from the participant were carried forward as the missing observations, with the aim of optimizing analysis and reducing bias associated with missing data [12]. During the course of data collection, six participants missed scheduled follow-up appointments and required one set of imputed data (over weeks 2-5) only. Descriptive statistics were used to explore weekly toxicity levels and patient-reported barriers to oral intake. Non-parametric correlations (Spearman rho) were conducted to determine associations between weekly toxicity scores and toxicity in week 5 of treatment. One-way repeated-measures ANOVA was used to record change over time points with ratio data (weight), with repeated-measures t tests used for post-hoc comparisons. For all statistical comparisons, p
< 0.05 was taken to indicate statistical significance.
Result
Toxicity severity during treatment
All participants experienced levels of toxicity of grade 1 or higher during treatment. For the majority of participants, peak toxicity was rated as grade 2 in severity (Table 3). The highest incidence of grade 3 toxicity was observed for mucositis, with small numbers of participants reported to have grade 3 toxicity for dysphasia and erythema (Table 3). No patient developed grade 3 acute toxicity for xerostomia or laryngeal edema, and no patients developed grade 4 toxicity.
Table 3: Peak incidence of toxicity at any stage during AFRT-CB
| Toxicity |
Grade 3 |
Grade 2 |
Grade 1 |
No toxicity |
| Erythema |
15% |
39% |
39% |
8% |
| Xerostomia |
0% |
77% |
23% |
0% |
| Dysphagia |
23% |
62% |
15% |
0% |
| Laryngeal edema |
0% |
15% |
31% |
54% |
| Mucositis |
31% |
62% |
0% |
8% |
Patterns of acute toxicity
The toxicity profile of participants from week 1 to four weeks post-treatment is reported in Figure 1. The onset of acute toxicity occurred in week 1. Except for laryngeal edema and erythema, the majority of participants had developed grade 1 or 2 toxicity by week 2. The first recording of grade 3 toxicity was found for mucositis at week 1 (8%), dysphasia at week 2 (8%), and erythema at week 5 (14%). The highest incidence and severity across acute toxicities was found in week 5, with peak incidence and severity evident for dysphagia, xerostomia, and mucositis. All patients had developed salivary changes by week 5. Recovery from acute toxicity had begun by four weeks post-treatment, with both severity and incidence reduced compared with week 5 of treatment across all toxicities scored.
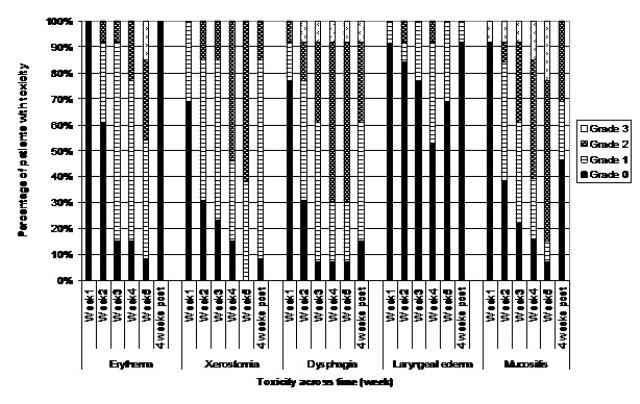
Figure 1 Percentage of AFRT-CB patients with acute toxicity as scored by CTCAE v.3 over time
Correlations between overall toxicity scores from weeks 1-4 and week 5 revealed no relationship between the toxicity profile at week 1 and toxicity at week 5 (p = 0.05, p = 0.71). In comparison, significant but moderate correlations were found for toxicity levels between week 2 and 5 (p = 0.34, p = 0.004), week 3 and 5 (p = 0.58, p
< 0.001) and week 4 and 5 (p = 0.66, p < 0.01).
Barriers to oral intake
During treatment, patients reported odynophagia, xerostomia, dysgeusia, anorexia, and oral ulceration as the most common barriers to oral intake, ranging in peak incidence from 39 -100% of the group (Table 4). The number of perceived barriers increased as treatment progressed, with the onset of reporting as early as weeks 1 and 2 of treatment (Figure 2). Week 3, however, was the time point when the incidence of patient-reported barriers to oral intake increased considerably (Table 4). In week 4, with the onset of twice daily fractions, 100% reported odynophagia, and two thirds reported taste alterations and xerostomia as barriers to oral intake. In week 5, odynophagia remained a concern for all with peak incidence for anorexia, dysgeusia, early satiety, nausea, constipation, oral ulceration, and xerostomia also observed at this time.
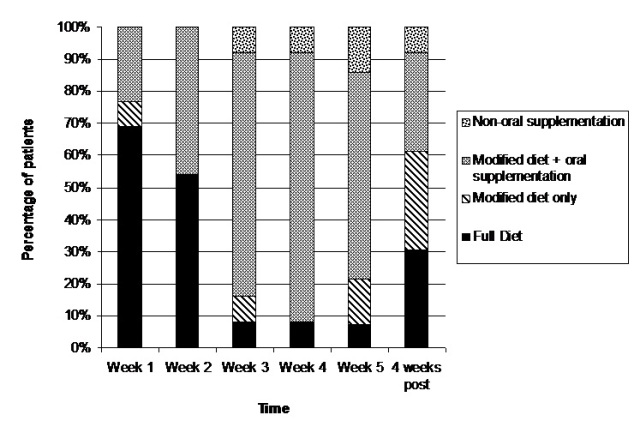
Figure 2: Pattern of most common patient-reported barriers to oral intake during AFRT-CB treatment
Table 4: Percentage of patients receiving AFRT-CB who reported listed side effects as barriers to oral intake
Patient-reported
side effect |
% reporting
side effect at
week 1 |
% reporting
side effect at
week 2 |
% reporting
side effect at
week 3 |
% reporting
side effect at
week 4 |
% reporting
side effect at
week 5 |
| Fatigue |
-* |
- |
8 |
8 |
- |
| Anorexia |
15 |
23 |
31 |
39 |
54 |
| Dysgeusia |
- |
54 |
54 |
62 |
77 |
| Early satiety |
- |
- |
- |
- |
8 |
| Nausea |
- |
- |
8 |
8 |
23 |
| Vomiting |
- |
- |
- |
15 |
8 |
| Diarrhoea |
- |
15 |
23 |
8 |
- |
| Constipation |
- |
- |
8 |
8 |
31 |
| Candidiasis |
8 |
8 |
15 |
8 |
8 |
| Ulceration |
8 |
- |
39 |
31 |
39 |
| Odynophagia |
23 |
69 |
92 |
100 |
100 |
| Xerostomia |
31 |
85 |
39 |
69 |
85 |
| Trismus |
- |
- |
8 |
8 |
8 |
*– refers to no patient reporting the listed side effect
Impact on functional swallowing and weight
Diet and fluid tolerance followed a similar pattern to clinician-rated dysphagia toxicity. Almost all participants required a modified diet with oral supplementation by week 3 as a result of toxicity and reported barriers to oral intake (Figure 3). Between week 3 and week 5, only one patient was able to tolerate a full diet and thin fluids, and by week 5 two patients required nasogastric tube insertion due to toxicity and concerns regarding aspiration. In week 5, two participants on modified diets with recommendations for oral supplementation were unable to tolerate the supplements due to oral burning/pain on consumption, and therefore were restricted to a modified diet only. On average, mean LOW for the cohort was 5kg during treatment, and a further 5kg between treatment completion and 1 month post-treatment, although this was not significant (F = 0.38, p = 0.89). Mean weight for the cohort at four weeks post-treatment was 73kg (SD = 18.56).
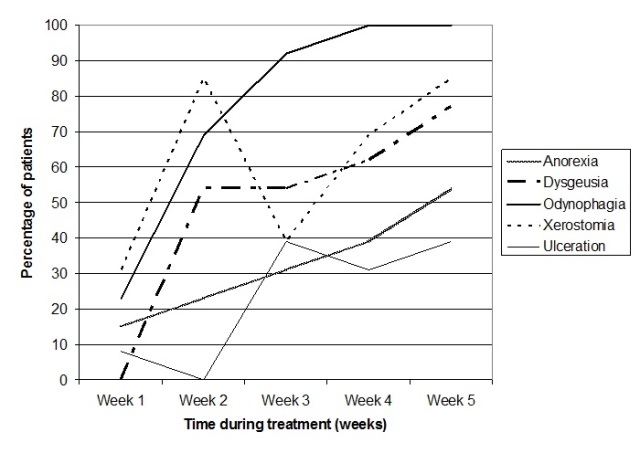
Figure 3 Pattern of diet and nutritional supplementation for AFRT-CB participants during and at 4 weeks post-treatment
Discussion
Previous reports have documented small numbers (1-14%) of patients developed acute grade 4 toxicity following AFRT-CB [2, 7] however none were recorded in the current cohort. Levels of grade 3 dysphagia and mucositis toxicity for the current cohort was similar to previously reported for rates of dysphagia toxicity (20-29%) and slightly less than that for mucositis (35-47%) [2, 7-9]. The majority of the current cohort developed mild-moderate toxicity similar to previous reports [9, 8, 7, 2], except for dysphasia. Grade 1 or 2 dysphasia toxicity has previously ranged from 42-68%, however in our cohort 77% developed grade 1 or 2 dysphasia toxicity.
The progression of acute toxicity following an AFRT-CB cohort has not yet been fully reported. From week 2, the toxicity profile correlated with toxicity at week 5 of treatment, indicating a relationship between early onset toxicity and the progression of toxicity in the final week of treatment. Knowledge of this relationship may assist clinicians to better educate patients regarding the course of their toxicity, as well as foresee potential need for supportive care in the end stages of treatment. Other accelerated fractionation regimens have found acute toxicity occurred earlier with greater severity when compared to conventional fractionation [13-15]. Our peak incidence for mucositis of 92% at week 5 is higher and occurred later than that found for patients who received a continuously hyper fractionated accelerated radiotherapy (CHART) regimen [15]. The current data were not dissimilar to reports of conventional treatment where peak toxicity occurred in the final 2 weeks of treatment in 85% of patients[16]. It is also possible that some individuals may have experienced a further increase in acute toxicity beyond week 5 of treatment, as some have reported that the most severe toxicity following AFRT occurred 5-7 weeks from the commencement of treatment [16, 14]. Although the current study did not follow participants beyond week 5, this potential for further deterioration must be acknowledged.
Clinician-rated toxicity provides an overall summary of the tolerance of radiotherapy treatment, however there is growing interest in the patient-experience and impact on function during and following treatment. Patient-reported barriers to oral intake were first recorded in weeks 1 and 2 in the current cohort, with the onset of mild to moderate dysphasia, xerostomia, and mucosal toxicity. Both clinician-rated toxicity and patient-reported barriers of odynophagia, xerostomia, dyspepsia, anorexia, and oral ulceration led to negative functional consequences with modifications to diet occurring for nearly 50% of participants in week 2, and 92% in week 3. Saunders et al. [15] found a larger proportion of patients undergoing a CHART treatment protocol described early (week 3) dysphasia and odynophagia, requiring analgesia, compared with patients receiving conventional treatment. However no comment was made regarding oral intake, need for nutritional supplementation, or nutritional outcomes. In the current cohort, similar to Saunders et al. [15], week 3 marked the increase of reported barriers to oral intake, and this co-occurred with 92% patients requiring a modified diet or nasogastric feeding. This result supports the need for early symptom management, swallowing and nutritional intervention.
The peak incidence for patient-reported barriers to oral intake occurred at week 5, however, similar to toxicity, it could be possible that the incidence of barriers to oral intake may continue to increase in the early weeks post-treatment. Supportive intervention from speech pathology and dietetics between treatment completions until four weeks post-treatment varied, due to patient need or clinical follow-up being determined by local centers. The few authors who have reported weekly assessment of toxicity until peak toxicity subsided did not comment regarding return to oral intake and weight maintenance [9, 6]. It is therefore difficult to extrapolate whether regular follow-up early post-treatment resulted in improved functional outcomes in these studies. Perhaps reduced support via contact with health professionals in the early post-treatment phase explains the continued weight loss post-treatment in the current cohort. These results suggest that the provision of supportive intervention for AFRT-CB patients needs to occur routinely pre-treatment, and on a weekly basis until the resolution of side effects, to ensure the barriers to oral intake are minimized and negative nutritional sequelae are avoided.
Conclusion
This is the first exploration of the pattern of onset and progression of early toxicity, patient-reported barriers to oral intake, functional swallowing, and weight in a cohort of oropharyngeal patients treated with AFRT-CB. Our findings suggest that mild-moderate toxicity impacting on oral intake begins in weeks 1 and 2 of treatment, with increasing severity and incidence by week 3 of treatment, prior to patients commencing their twice daily “concomitant boost” treatments. The most severe toxicity was observed in the final week of treatment and it is possible, that for some, severe toxicity may have continued into the post-treatment phase. The requirement of a modified diet and weight loss continues for the majority of patients at four weeks post-treatment despite improvement in acute toxicity. The limitations of this research are recognized and include small participant numbers limiting the generalizability of our results and a single time point post-treatment record of toxicity, rather than regular assessment to accurately record resolution of acute toxicity in the early post-treatment phase. Future research needs to examine the resolution of acute toxicity and barriers with a routine approach. Additionally, clinicians grading acute toxicity and providing supportive care should be aware of the patient-perspective regarding their treatment experience. The reporting of barriers to oral intake undoubtedly relates to patient motivation, compliance, and family support; a topic requiring exploration in future studies.
Learning Points
- Mild to moderate acute toxicity associated with altered fractionation radiotherapy impacts on swallowing and nutrition from week one of treatment.
- A modified diet and nutritional supplementation is required by almost all patients receiving altered fractionation radiotherapy by week 3 of treatment, and is required for several weeks following treatment.
- Patient-reported barriers to oral intake add a valuable insight to assist with providing supportive care to patients through altered fractionation radiotherapy.
Authors' Contribution
BC: was primarily responsible for the concept and design of the study, participant recruitment, data collection, analysis and interpretation, reviewing the literature, and drafting and writing the article.
PC, EW: contributed to the concept and design of the study, specialist knowledge regarding interpretation of data and manuscript preparation, and reviewing drafts of writing.
WD: contributed specialist dietetic knowledge, was primarily involved in nutritional endpoint data collection, and reviewing drafts of writing.
SP: contributed to the concept and design of the study, specialist knowledge regarding radiotherapy toxicity and its data collection and reviewing drafts of writing.
We acknowledge all authors have contributed significantly to the preparation of this manuscript and all are in agreement with the content presented herein.
Conflict of Interests
None declared
Ethical Considerations
The study was approved by the institute review board
Funding
Cancer Collaborative Group at the Princess Alexandra Hospital, and the Cancer Council Queensland, Australia
Acknowledgement
The onset and progression of acute toxicity and patient-reported side effects during altered fractionation radiotherapy with concomitant boost (AFRT-CB) for oropharyngeal cancer, Tri-Society Head & Neck Oncology Meeting, Singapore 2011
References
[1]. Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot JC, Le Maitre A, Pajak TF, Poulsen MG et al., Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006; 368 (9538):843-854. [Pubmed].
[2]. Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, Garden AS, Ridge JA, Cooper JS, Ang KK. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000; 48 (1):7-16. [Pubmed].
[3]. Allal AS, Bieri S, Miralbell R, Marchal F, Lehmann W, Kurtz JM. Feasibility and outcome of a progressively accelerated concomitant boost radiotherapy schedule for head and neck carcinomas. Int J Radiat Oncol Biol Phys 1997; 38 (4):685-689. [Pubmed].
[4]. Ang KK, Peters LJ (1992) Concomitant boost radiotherapy in the treatment of head and neck cancers. Semin Radiat Oncol 2 (1):31-33
[5]. Knee R, Fields RS, Peters LJ. Concomitant boost radiotherapy for advanced squamous cell carcinoma of the head and neck. Radiother Oncol 1985;4 (1):1-7. [Pubmed].
[6]. MacKenzie R, Balogh J, Choo R, Franssen E. Accelerated radiotherapy with delayed concomitant boost in locally advanced squamous cell carcinoma of the head and neck. International Journal of Radiation Oncology Biology Physics 1999; 45 (3):589-595. [Pubmed].
[7]. Ghoshal S, Goda JS, Mallick I, Kehwar TS, Sharma SC. Concomitant boost radiotherapy compared with conventional radiotherapy in squamous cell carcinoma of the head and neck: A phase III trial from a single institution in India. Clin Oncol 2008; 20 (3):212-220. [Pubmed].
[8]. Morris MM, Schmidt-Ullrich RK, DiNardo L, Manning MA, Silverman L, Clay L, Johnson CR, Amir C. Accelerated superfractionated radiotherapy with concomitant boost for locally advanced head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 2002; 52 (4):918-928. [Pubmed].
[9]. Olmi P, Crispino S, Fallai C, Torri V, Rossi F, Bolner A, Amichetti M, Signor M, Taino R, Squadrelli M, Colombo A, Ardizzoia A, Ponticelli P, Franchin G, Minatel E, Gobitti C, Atzeni G, Gava A, Flann M, Marsoni S. Locoregionally advanced carcinoma of the oropharynx: Conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy - A multicenter randomized trial. International Journal of Radiation Oncology Biology Physics 2003; 55 (1):78-92. [Pubmed].
[10]. Trotti A. Toxicity in head and neck cancer: A review of trends and issues. International Journal of Radiation Oncology Biology Physics 2000; 47 (1):1-12. [Pubmed].
[11]. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3. 0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13:176-181. [Pubmed].
[12]. Lachin JM. Statistical considerations in the intent-to-treat principle. Control Clin Trials 2000; 21 (3):167-189. [Pubmed].
[13]. Denham JW, Walker QJ, Lamb DS, Hamilton CS, O'Brien PC, Spry NA, Hindley A, Poulsen M, O'Brien M, Tripcony L. Mucosal regeneration during radiotherapy. Radiother Oncol 1996; 41 (2):109-118. [Pubmed].
[14]. Kaanders JHAM, Van Daal WAJ, Hoogenraad WJ, Van der Kogel AJ. Accelerated fractionation radiotherapy for laryngeal cancer, acute, and late toxicity. Int J Radiat Oncol Biol Phys 1992; 24 (3):497-503. [Pubmed].
[15]. Saunders MI, Dische S, Barrett A, Parmar MKB, Harvey A, Gibson D. Randomised multicentre trials of CHART vs conventional radiotherapy in head and neck and non-small-cell lung cancer: An interim report. Br J Cancer 1996; 73 (12):1455-1462. [Pubmed].
[16]. Horiot J, Bontemps P, Van den Bogaert W, Le Fur R, van den Weijngaert D, Bolla M, Bernier J, Lusinchi A, Stuschke M, Lopez-Torrecilla J et al., Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: Results of the EORTC 22851 randomized trial. Radiother Oncol 1997; 44 (2):111-121. [Pubmed].


