Research

Matrix metallopeptidase 9 (MMP-9) expressions in oral cancer and adjacent normal tissue
1Devendra Kumar Ravi, 3Mohan Kumar, 2Gajendra Singh 6 Shyam Bahadur Rai5Tanvi Chincholkar, 4,7 ,Ajit Kumar Saxena,1,5,8 Manoj Pandey
- 1Department of Surgical Oncology,
- 2Anatomy
- 3Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221 005, India
- 4,Centre for Experimental Medicine and Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221 005, India
- 5National Institute of Research in Environmental Health, Bhopal, India
- 6Department of Physics, Banaras Hindu University, Varanasi 221 005, India
- 7Presently working at Department of Pathology & Lab Medicine, All India Institute of Medical Sciences, Patna, Bihar
- 8Presently working at Surgical Oncology, Bhopal Memorial Hospital and Research Centre, Bhopal, India
- Submitted:Tuesday, May 13, 2014
- Accepted: Saturday, December 13, 2014
- Published:Monday, December 15, 2014
This is an Open Access article distributed under the terms of
the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/3.0) which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Background
Despite advances the oral cancer in India is continued to be diagnosed in late stages and have poor prognosis. The disease has preponderance for local recurrence and development of second primaries. To explain this theory of field cancerization has been proposed. In this study we looked at the expression of MMP-9 in tumor tissue and in the adjacent microscopically normal tissue, to explain higher failures in oral cancer after successful treatment.
Patients and Methods
MMP-9 expression was looked at by immunohistochemistry in 100 patients with oral cancer in the tumor and adjoining normal tissue. Data on stage of the disease, treatment and other demographic and pathological features was collected in presetproforma.
Results
Frequent expression of MMP-9 was observed in Oral Squamous Cell Carcinoma (OSCC), with 85% and 80% of tumor and adjacent tissue samples expressing the protein. The expression in patients with neck node metastasis was significantly higher compared to those with no nodal metastasis.
Conclusions
The results of the present study show that MMP-9 overexpression is a good indicator of aggressive nature of the disease and is associated with increased nodal metastasis.
Introduction
In oral squamous cell carcinoma, poor prognosis is related with loco-regional recurrence and metastatic disease. Positive histological margins, when reported, are associated with the local recurrent disease. However, failures have been observed in surgical negative margin due to lack of sensitivity in evaluation of margin, despite high specificity[1 2]. This has led to identification of cells, at the surgical negative margin, that have transformed into the cancer cell genetically but, not expressed phenotypically. Such genetically transformed cells could not be identified in surgical margin on histological assessment, and later, progress to produce recurrent disease. The role of genetic alteration at the molecular level has been investigated and several molecular factors, such as, TP53 mutations, VEGF and MMP-9 expression, telomerase activity and microsatellite instability, which alter the prognosis in the margin negative cases have been identified [3 4].
One important hallmark of cancer progression is the degradation of the extracellular matrix (ECM), which allows cancer cells to invade the surrounding tissue. Matrix metalloproteinases(MMPs) are comprise a group of structurally related zinc-dependent endopeptidases that play major roles in tissue regeneration [3 6] and are of importance in virtually any process that involves remodeling, ranging from angiogenesis and cell proliferation to wound healing and degradation of dysfunctional tissue, and also in pathological processes such as inflammation, tumor invasion and metastasis[7].
Matrix metalloproteinase-9 (MMP-9), a 92 kDa gelatinase, that plays important roles in tumor invasion and angiogenesis [8 9], and is associated with the aggressive nature of many cancers, including OSCC. It is thought to cause type IV collagen degradation, a main component of basement membranes [10]. Increased MMP-9 protein levels, due to transcriptional upregulation of MMP-9, leads to increased MMP-9 activity.MMP expression increases a chance for local relapse, and lymph node and distant metastasis. High MMP-9 expressions are associated with the progression of lymph-node spread which is correlated with poor disease free survival []. However, no correlation between MMP9 expression with clinical variables, such as, tumor stage and recurrence rate has been observed [5 6]. It has been suggested that MMP-9 expression at histologically negative surgical margins could predict OSCC recurrence [11].
In this study, the ability of MMP-9 expression in identifying tumor-related alterations in histologically negative surgical margins has been evaluated. In this way, it is hypothesized that the expression profile of this gene in histologically negative margins could act as a more sensitive and useful marker for the detection of molecular alterations associated with local disease control in OSCC patients.
Material and Methods
Patient and Sample Selection
A cohort of 100 patients was selected for the purpose of study. These patients were diagnosed with primary HNSCC at the Department of Surgical Oncology, IMS, BHU, during 2006-2012, and were treated withsurgery as primary curative modality of treatment. In all the cases, the surgical margins were reported to be histological negative by histopathologist at Department of Pathology, IMS, and BHU. Tumour tissuespecimens from various sites in the oral cavity were obtained, along with the histological negative surgical marginsfrom clinically normal mucosa 1 cm from the tumor (Table 1).
Table 1: Sites for Sample Collection
|
S.No.
|
Site
|
Number of Samples
|
|
1.
|
Buccal mucosa (Cheek)
|
44
|
|
2.
|
Tongue
|
12
|
|
3.
|
Alveolus
|
19
|
|
4.
|
Lip
|
14
|
|
5.
|
GingivoBuccal Sulcus (G. B. Sulcus)
|
11
|
Immunohistochemistry
Immunohistochemical staining technique was carried out to determine the MMP-9 protein expression levels in tumour specimens and respective surgical margins. Deparaffinzed slides of 4-6 micron thick tissue sections were subjected to antigen retrieval in sodium citrate buffer (pH 6.0) with microwave irradiation for 10 minutes. Endogenous peroxidase activity was then quenched incubating specimens in peroxidase block for 5minutes.Prior to application of primary antibodies, the sections were incubated with serum block for 20 minutes. Diluted primary antibody (1:200µl) (HPA001238 Sigma Aldrich) was then added to specimen and sections incubated for 20 minutes at room temperature. Washed specimens were then incubated with secondary antibodyfor about 30 minutes. Immunohistochemical reactions were developed by addition of HRP-Streptavidin complex followed by HRP substrate, resulting in brown staining of specimens. After counter-staining with haematoxylin, the sections were observed under light microscope and the staining was graded and scored. Also, the type of staining was recorded.
Statistical Analysis
Statistical analysis of the obtained results was carried out using Fisher’s Exact Test using GraphPadQuickcalcs software, available online, in order to determine any correlation between the expression of MMP-9 protein and various findings in the OSCC.
Results
Frequent expression of MMP-9 was observed in Oral Squamous Cell Carcinoma (OSCC), with 85% and 80% of tumor and adjacent tissue samples expressing the protein ((Figure 1 2 3 4). With respect to the tumor grade,the expression in adjacent tissuewas decreased in well differentiated tumors, while observed to be increased in case of poorly differentiated tumors (Figure2 3 4). Increasedimmunohistochemical expression of MMP-9 was observed in tongue OSCC at the invasion front.
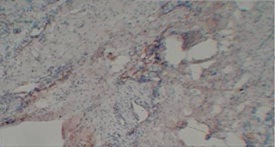
Figure 1: Photomicrograph showing negative staining for MMP9
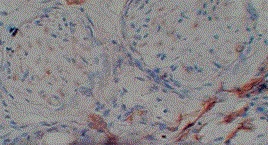
Figure 2: Photomicrograph showing cytoplasmic 1+ expression of MMP 9 in grade I squamous carcinoma (x100)
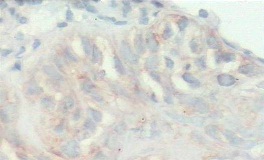
Figure 3: Photomicrograph showing moderate cytoplasmic (2+) expression of MMP 9 in moderately differentiated squamous cell carcinoma (x100)
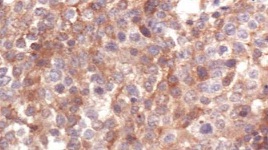
Figure 4: Photomicrograph showing 3+ cytoplasmic expression of MMP9 in squamous cell carcinoma.
MMP-9 expression was significantly higher (p<0.0001) in patients with regional lymph node metastasis compared to those with non-metastatic tumors. All cases with lymph node negative disease were negative for MMP-9 expression. [Table 2 ]
|
Variable
|
MMP-9 Positive TumorSamples
|
MMP-9 Negative TumorSamples
|
MMP-9 Positive Adjacent TissueSamples
|
MMP-9 Negative Adjacent Tissue Samples
|
P value
|
|
Overall
|
|
|
85
|
15
|
80
|
20
|
NS
|
|
Tumor Grade
|
|
|
Well differentiated
|
43
|
15
|
34
|
20
|
|
|
Moderately differentiated
|
32
|
28
|
|
|
Poorly differentiated
|
10
|
18
|
|
|
Lymph Node Metastasis
|
|
|
Lymph node positive
|
75
|
10
|
70
|
15
|
<0.0001
|
|
Lymph node negative
|
-
|
15
|
-
|
15
|
Discussion
It has been supposed that tumor cells undergoing genetic alteration in surgical margins reported negative by the histologist are responsible for tumor recurrence and prognosis of the patient in head and neck squamous cell carcinoma. In this study, we have demonstrated the overexpression of MMP-9 in the tumor cell and adjacent negative margin in the surgically treated case of oral squamous cell carcinoma and its correlation with lymph node metastasis. The MMP-9 over expression (in the tumor cell) has been well correlated with the lymphnode metastasis [5 6]. The patients having positivecervical lymph nodes with adjacent tissue reported normal were also investigated for the higher expression of MMP-9. These are the cells which are probably responsible for the local or lymph node recurrence. Many other studies have reported high MMP-9 expression associated with the progression of lymph node spread, which are correlated with poor disease free survival (DFS). It has been shown that increased expression of this gene leading to enhanced synthesis of this protein (MMP-9), independently on other factors, increases a chance for local relapse, and distant metastasis. Consequently, patients with oral cancer have poor disease-free survival, as well as poor overall survival. No statistical correlation was found between the level of MMP-9 expression and histological and nuclear grade, tumor size, disease relapse or patients overall survival.
Our results of expression of MMP-9 in tumor tissue are comparable to the literature and investigation of positive expression of MMP-9 in adjacent normal tissue throws new light in explaining the cause of the recurrence of oral cancer even when reported negative by the pathologist. Thus, MMP-9 expression in adjacent normal tissue may play an important role in progression of HNSCC. Many other researchers have also demonstrated that overexpression of tumor-related genes in histologically negative surgical margins is a frequent event by the use of quantitative RT–PCR and may be an useful tool in detecting actually negative HNSCC surgical margins and the overexpression of specific genes in these margins could be helpful in the identification of patients with a higher risk of developing second primary tumor and local recurrences [3 11].
Conclusion
Based on these results, we may speculate that the expression of these genes in HNSCC surgical margins, diagnosed as tumor-free by conventional histopathology, could be a helpful biomarker to identify subjects at risk of new disease. It can also be used as an alternative tool to guide the surgeon in the delineation of the HNSCC resection extent and also during follow-up in the planning of adjuvant therapy.
Authors' Contribution
DKR: Literature search and preparation of manuscript.
MK: Editing the manuscript and interpretation of pathological results
GS: Concept and design, editing of manuscript.
SBR: Concept and design, editing of manuscript.
TC: Literature search and preparation of manuscript.
AKS: Concept and design, editing of manuscript.
MP: Concept and design, editing of manuscript.
Conflict of Interests
The authors declares that there are no conflicts of interests.
Ethical Considerations
The study was approved by the Institute Ethics Committee.
Funding
None
References
[1]Kowalski L.P, Carvalho AL, Martins Priante AV, Magrin JPredictive factors for distant metastasis from oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2005; 41(5): 534-541.[PMID: 15878760
[Pubmed]
[2]Larsen S. R, Johansen J, Sørensen JA, Krogdahl A. The prognostic significance of histological features in oral squamous cell carcinoma. JOralPatholMedicine. 2009; 38(8): 657-662[PMID: 19563504][Pubmed]
[3].Decarvalho A.C, Kowalski LP, Campos AH, Soares FA, Carvalho AL, Vettore ALClinical significance of molecular alterations in histologically negative surgical margins of head and neck cancer patients. Oral Oncol. 2012; 48(3):240-8. [PMID: 22104250][Pubmed]
[4].Garnis C, Buys TP, Lam WL..Genetic alteration and gene expression modulation during cancer progression. Mol. Cancer 2004; 3:, 3-9. [PMID: 15035667
[Pubmed]
[5].. Vilen S.V, Salo T, Sorsa T, Nyberg P.Fluctuating Roles of Matrix Metalloproteinase-9 in Oral Squamous Cell Carcinoma. Scientific World Journal. 2013; 2013:920595 [PMID: 23365550][Pubmed]
[6]De Vicente J.C, Fresno MF, Villalain L, Vega JA, Hernández Vallejo G. Expression and clinical significance of matrix metalloproteinase-2 and matrix metalloproteinase-9 in oral squamous cell carcinoma. Oral Oncol 2005; 41(3): 283–293. [PMID: 15743691][Pubmed]
[7].Nagase H, VisseR,Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006; 69: 562‐73. [PMID: 16405877][Pubmed]
[8].Chen W, Fu X, Ge S, Sun T, Sheng Z. Differential expression of matrix metalloproteinases and tissue-derived inhibitors of metalloprotein¬ase in fetal and adult skins. Int J Biochem Cell Biol 2007;39(5):997- 1005. [PMID: 17409012][Pubmed]
[9]Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997; 277(5323):225-8. [PMID: 9211848][Pubmed]
[10].Stetler-Stevenson WG, Aznavoorian S, LiottaLA..Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993; 9:541–573. [PMID: 828047
[Pubmed]
[11]..Ogbureke K U, Weinberger PM, Looney SW, Li L, Fisher LW.Expressions of matrix metalloproteinase-9 (MMP-9), dentin sialophosphoprotein (DSPP), and osteopontin (OPN) at histologically negative surgical margins may predict recurrence of oral squamous cell carcinoma,” Oncotarget. 2012; 3(3):286–298. [PMID: 22410369][Pubmed]


