Research

Extent of Disease on Initial Bone Scan Predicts Survival among Breast Cancer Patients with Bone Metastasis.
1,Takeshi Nagashima, 1Masahiro Sakakibara, 1Takafumi Sangai, 1Hiroshi Fujimoto, 2Takishima, 3Yukio
Nakatani, 1Masaru Miyazaki
- 1Department of General Surgery,
- 2Department of Radiology,
- 3Department of Diagnostic Pathology, Chiba University Graduate School of Medicine, 1-8-1 Inohana, Chuo-ku, Chiba 260-0856, Japan.
- Submitted: May 7, 2014
- Accepted: June 06, 2014
- Published: June 10, 2014
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Introduction
Breast cancer is the most common cancer among women. It is important to distinguish subset of patients with aggressive disease at risk for early progression resulted in cancer death. The present study attempts to evaluate quantitatively the initial bone scan appearance among breast cancer patients with bone metastasis, and to clarify the correlation with patient’s outcome
Study design
Retrospective study in a single institute.
Material and Methods
The subjects consisted of 29 breast cancer patients with bone metastasis which developed after surgery. The cases recurred in other visceral sites at the same time of bone metastasis, were excluded from this series. On the basis of the extent of disease (EOD), the patients were divided into low-EOD and high-EOD groups, and their outcome was analyzed retrospectively. Statistical differences were determined by the Student’s t test for continuous variables and the chi-square test for categorical variables. Overall survival was evaluated by Logrank test.
Results
There was no significant difference between the groups concerning with the clinicopathological background. However, 5-year survival rate after recurrence among low-EOD group was 50.8%, which was significantly higher than high-EOD cases (20.9%, p=0.001).
Conclusions
This is the first report described the association between quantitative grading of bone metastases and their survival after recurrence among breast cancer patients with bone metastasis. The use EOD grade on initial bone scan is useful for the prognostic prediction, which required only a simple procedure without expensive or complicated equipment.
Key words
Breast cancer, Bone metastasis, Prognosis, Scintigraphy
Introduction
Breast cancer is the most common cancer among women. Despite advances in treatment of breast cancer, patients remain at risk for recurrence for many years even after receiving standard treatment. Several studies reported that 47 to 60% of breast cancer patients with distant metastatic disease had a bone metastasis at diagnosis or subsequently developed a bone metastasis [1,2]. Bone metastasis causes skeletal-related events (SREs), including pathological fractures, spinal cord compression, hypercalcemia, and surgical operation or irradiation to the metastatic lesion, which often worsen quality of life significantly. Furthermore, having a possible bone metastasis was associated strongly with mortality among women with breast cancer [3]. Hence it is important to distinguish subset of patients with aggressive disease at risk for early progression resulted in cancer death. The extent of bone metastases and survival rates has been investigated in prostate cancer patients in several studies. However, no publications are found in the field of metastatic breast cancer on this topic. The present study attempts to evaluate quantitatively the initial bone scan appearance among breast cancer patients with bone metastasis, and to clarify the correlation with patient’s outcome.
Material and Methods
The subjects consisted of 29 breast cancer patients with bone metastasis which developed after surgery. The cases recurred in other visceral sites at the same time of bone metastasis, were excluded from this series. All were female and had no previous history of malignant disease. All the patients received breast surgery from 2000 to 2011 at our hospital. Clinically node-negative patients received sentinel node biopsy. When the sentinel node was noted to contain malignant cells, a complete axillary lymph node dissection was performed subsequently. The patients with tumor free sentinel nodes on intraoperative investigations underwent no further axillary surgery. Hormone receptor status and human epidermal growth factor receptor type-2 (HER2) overexpression were assessed by immunohistochemical staining. All the patients treated with partial resection of the breast received whole breast irradiation after surgery. Systemic adjuvant therapy was performed according to their clinicopathological diagnosis. Hormonal treatment was recommended to positive estrogen receptor (ER) cases, which consisted of tamoxifen for premenopausal and aromatase inhibitors for postmenopausal patients. Trastuzmab was used for the cases with HER2 overexpression. Bone scintigraphy was carried out annually on month same as their operations were performed, and when the patient became symptomatic with bone pain. After a diagnosis of bone metastasis, a bone-modifying agent was used with anticancer drugs. For the assessment of recurrences and therapeutic effect, magnetic resonance imaging and computed tomography were also applied.
The whole body bone scan images were acquired within 3–5 hours after intravenous injection of 555 MBq 99mTechnetium-hydromethylene diphosphonate (99mTc-HDMP, Nihon Mediphysics Co. Ltd, Japan), using a gamma camera equipped with a low-energy, high-resolution collimator (Infina Hawkeye 4, GE Medical Systems, Inc., USA). Both anterior and posterior images were recorded at a speed of 13 cm/min and 256 x 1024 matrix. Energy discrimination was provided by a 20 % window centered on the 140 keV of 99mTc. On the basis of the number or extent of metastases, the scans were divided into five extent of disease (EOD) grades defined by Soloway et al. [4] as follows: grade 0; normal or abnormal due to benign bone disease, 1; number of bony metastases less than six, each of which is less than 50% the size of a vertebral body (one lesion about the size of a vertebral body would be counted as two lesions), 2; number of bone metastases between six and 20, size of lesions as described above, 3; number of metastases more than 20 but less than a “super scan”, and 4; “superscan” or its equivalent, ie., more than 75% of the ribs, vertebrae. and pelvic bones (Table 1). The correlation between the EOD grades and the patient’s outcome was analyzed retrospectively. The study was approved by our institutional review board, and all patients provided written informed consent. Statistical differences were determined by the Student’s t test for continuous variables and the chi-square test for categorical variables. Kaplan-Meier curves were generated for presenting overall survival and evaluated by Logrank test. P-values of less than 0.05 were considered to indicate statistically significant differences.
|
Glade
|
Bone scan findings
|
|
0
|
normal or abnormal
due to benign bone disease
|
|
1
|
number of bony
metastases less than six, each of which is less than 50% the size of a
vertebral body
|
|
2
|
number of bone
metastases between six and 20
|
|
3
|
number of
metastases more than 20 but less than a “super scan”
|
|
4
|
“superscan” or its
equivalent, ie., more than 75% of the ribs, vertebrae. and pelvic
bones
|
Results
The patients' ages ranged from 33 to 67 years with an average of 50.6 years. The mean maximum breast tumor dimension was 3.3 cm, ranging from 0.8 to 6.0 cm. Twenty cases (68.9%) had positive ER status and six cases (20.7%) presented overexpression of HER2. Ten patients received preoperative chemotherapy with anthracycline containing regimen followed by taxan. Breast surgeries were performed for all patients, which consisted of mastectomy in 16 cases (55.2%) and breast conservative surgery in the remaining 13 cases. Twenty-three cases (79.3%) underwent axillary lymph node dissection and 22 cases had lymph node involvements.
The patients were classified as EOD grade 0 (n=2), grade 1 (n=9), grade 2 (n =14), grade 3 (n=2) and grade 4 (n=2), as well as divided into low-EOD (grade 0-1, n=11) and high-EOD (grade 2-4, n=18) groups [Figure 1a,,b,] There was no significant difference between the groups concerning patient’s age, tumor size, ER status, HER2 overexpression, nodal involvement and surgical procedure (Table 2)). The mean intervals between the operation and recurrence were 45 (5-128) months, in which there was no significant difference between 47 months in low-EOD and 39 months in high-EOD group. Cancer associated deaths were observed in eleven cases in the median 25 (5-128) months follow-up after recurrence. 5-year survival rate among low-EOD group was 50.8%, which was significantly higher than high-EOD cases (20.9%, p=0.001,
Figure 2)
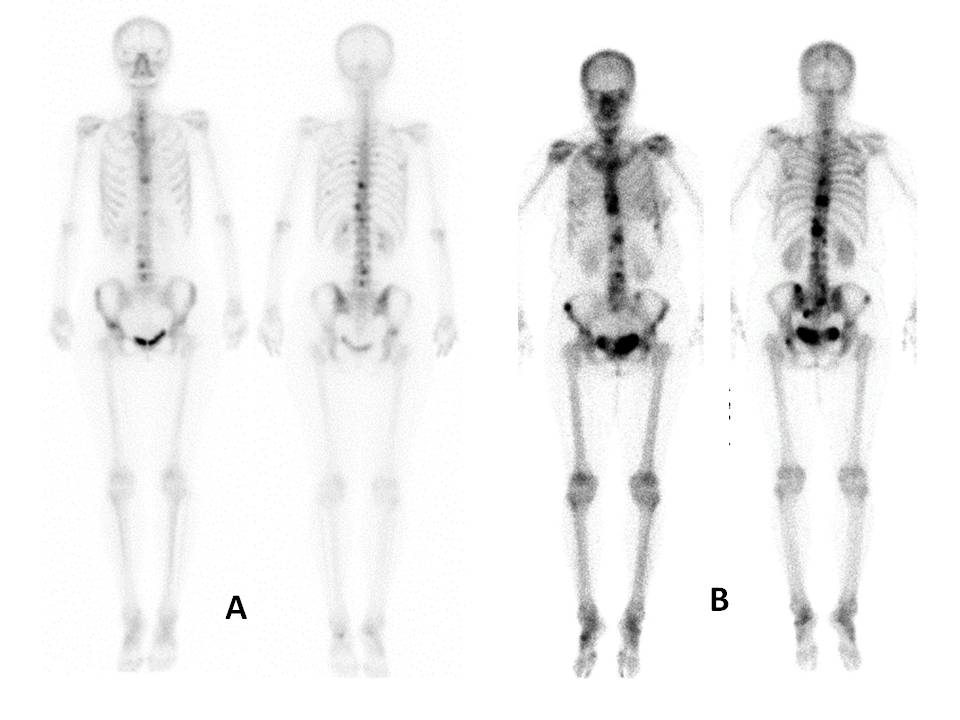
Fig.1: Bone scan images of the patients with bone metastases.
(a) EOD grade 1. Anterior and posterior views of bone scan showing four vertebral metastases, which is less than 50% the size of a vertebral body. (b) EOD grade 2. Several lesions involving sternum, vertebras and pelvic bones. Number of metastatic lesions is approximately 12.
|
|
Low-EOD
(n=11)
|
High-EOD
(n=18)
|
P-value
|
|
Age (y)
|
49 +/- 9
|
51 +/- 9
|
0.366
|
|
Tumor size (cm)
|
3.4 +/- 1.4
|
3.2 +/- 1.3
|
0.428
|
|
ER (+/-)
|
7 / 4
|
13 / 5
|
0.628
|
|
HER (+/-)
|
4 / 7
|
2 / 16
|
0.103
|
|
Node metastasis
(+/-)
|
9 / 2
|
13 / 5
|
0.558
|
|
Bp / Bt
|
4 / 7
|
9 / 9
|
0.474
|
|
Axillary
dissection (+/-)
|
10 / 1
|
13 / 5
|
0.228
|
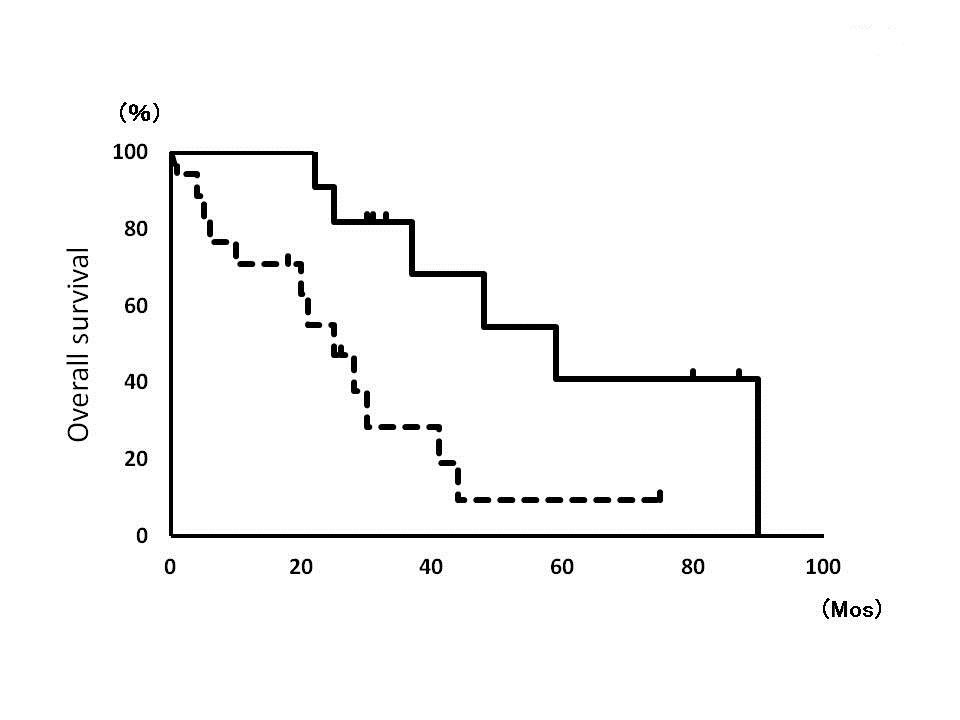
Fig. 2: Overall survival curves of low-EOD group (solid line) and high-EOD group (dotted line).
Discussion
Bone scintigraphy has been commonly used as a primary imaging procedure for detecting bone metastasis, and for monitoring the progression of bone metastases. Although the early recognition of metastasis will allow rapid institution of effective palliative treatment, the current guidelines do not recommend tests with CT or bone scintigraphy to detect asymptomatic metastatic and/or recurrent breast cancer [5]. However, the information on bone metastasis helps patients to understand their disease and SREs, and this knowledge would contribute to optimization of the treatment and care system for SREs. Bisphosphonates has been widely used for the reduction of SREs and the prolongation of event-free intervals. These drugs improve the quality of life of the patients and reduce bone pain. Furthermore, an activator of nuclear factor kappa-B ligand (RANKL) antibody (Denosumab) has recently shown superiority for the reduction of skeletal complications of metastatic bone disease compared with zoledronic acid [6]. For the staging and monitoring of tumor response to therapy, and for predicting the survival of patients with bone metastasis, a simple method to distinguish subset of patients with aggressive disease is needed.
However optimal method for detecting, quantifying, and grading tumor metastasis to bone is difficult, several trials have attempted to quantify bone metastasis among the patients with prostate cancer. In 1979, Hovsepian JA, et al. [7] evaluated 102 patients who died of cancer of the prostate. All had bone metastases at the time of diagnosis. Using plain radiographs of the chest, thoracic spine, lumbosacral spine, and pelvis, the extent of metastases in each of eight sites (ribs, lungs, shoulders, thoracic spine, lumbosacral spine, pubis-ischium, ilium, and proximal femurs) was determined as a percent involvement by a metastasis at a given radiologic site. They found that two radiographs, the posterioanterior chest and the pelvis film, were needed to subdivide all of the patients with metastatic prostate cancer into four radiologic risk groups. With the three sites of lung, pubis-ischium, and proximal femur, patients were ranked in order of increasing predictive death rates. The patient survival was markedly different, depending on the number of sites involved and the extent of involvement at each site. Soloway MS, et al. [4] analyzed the survival of 166 patients who were treated with various forms of androgen deprivation for metastatic prostate cancer. They used the EOD grading on the initial bone scan when bone metastasis was demonstrated, and reported that the EOD grade correlates with survival. The 2-year survival rates for EOD 1 to 4 were 94%, 74%, 68%. and 40%, respectively.
The EOD of bone metastases and survival rates have been investigated in prostate cancer patients. However, no publications are found in the field of metastatic breast cancer on this topic. In this series, we analyzed the bone scan images obtained from 29 breast cancer patients with bone metastasis, using EOD grades defined by Soloway MS, et al., and evaluated the correlation between the EOD grades and the patient’s outcome retrospectively. The EOD on initial bone scan correlated well with their survival. The 5-year survival rate among breast cancer patients with low-EOD was 50.8%, which was significantly higher than high-EOD cases (20.9%). This is the first report presenting the significant correlation between the bone scan images and prognosis among breast cancer patients with bone metastasis. This information would be useful when considering follow-up schedules and when designing therapies targeting metastatic breast cancer. Breast cancer patients with metastatic involvement usually have more than one disease site. In addition, the assessment of response and progression varies widely, and may be difficult to reproduce in a large population. However it was better to compare prognoses among the patients with 5 categories of EOD, we compelled to divide into the low and high EOD groups, because of the small number of cases. Further examination and prospective studies in a large number of patients should be required to clarify correlations in greater detail. Furthermore, to more accurately determine the amount of tumor present at baseline and to monitor the tumor’s response to therapy, for example, a computer-aided diagnosis system on bone scintigraphy images would be necessary for improving the interpretations of bone scan images, which can help with understanding accurately the EOD of bone metastases.
Conclusions
Our current series evaluated quantitatively the initial bone scan appearance among breast cancer patients with bone metastasis, and revealed the correlation with patient’s outcome even though small number of cases. This is the first report described the association between quantitative grading of bone metastases and their survival after recurrence. The use EOD grade on initial bone scan is useful for the prognostic prediction among breast cancer patients, which required only a simple procedure without expensive or complicated equipment.
List of abbreviations used
SRE, skeletal-related events; HER2, human epidermal growth factor receptor type-2; ER, estrogen receptor; 99mTc-HDMP, 99mTechnetium-hydromethylene diphosphonate; EOD, extent of disease;
RANKL: receptor activator of nuclear factor kappa-B ligand
Conflict of Interests
The authors have no conflicts of interest to declare.
Authors’ contributions
TN: conception and design, acquisition of data, analysis and interpretation of data, drafting and revising the article, responsible for drafting the manuscript.
MS,TS and HF: conception and design, acquisition of data, reviewed and drafted the manuscript.
HT: Radiologist, acquisition of data, reviewed and drafted the manuscript.
YN: Pathologist, acquisition of data, reviewed and drafted the manuscript.
MM: revising the manuscript and final approval for submission.
References
[1].. Scheid V, Buzdar AU, Smith TL, Hortobagyi GN. Clinical course of breast cancer patients with osseous metastasis treated with combination chemotherapy. Cancer 1986;58:2589-2593. [Pubmed]
[2]Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-1594.[Pubmed]
[3]Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, Blackburn J, Arora T, Kilgore ML. Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999-2006. Breast Cancer Res Treat 2012;131:231-238. [Pubmed]
[4]. Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, Moinuddin M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988; 61:195-202. [Pubmed]
[5]Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, Hantel A, Henry NL, Muss HB, Smith TJ, Vogel VG, Wolff AC, Somerfield MR, Davidson NE; American Society of Clinical Oncology. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:961-965
[6].Diel IJ. Bone Metastasis in Breast Cancer. Breast Care. 2012;7:90-91. [Pubmed]
[7]Hovsepian JA, Byar DP. Quantitative radiology for staging and prognosis of patients with advanced prostatic carcinoma. Correlations with other pretreatment characteristics. Urology 1979;14:145-150. [Pubmed]


