Research

Neutrophil-Elastase Inhibitor- Sivelestat Assists in Reducing Ischemia Reperfusion Injury in a Muscle Flap Model: An Experimental Study
1Zeynep Kamuran Sevim, 1Memet Yazar, 2Aköz Funda Saydam, 1Volkan Medeni Kıyak, 1Aysin Yeşilada Karasoy, 1Sami Samin, 3Şenay Yener, 1Dilgem Memmedov, 4Başak Erginel, 5Fatih A. Aydın
1Department of Plastic and Reconstructive Surgery, Sisli Etfal Research and Training Hospital, Istanbul, Turkey 2Department of Plastic and Reconstructive Surgery, Bagcilar Research and Training Hospital, Istanbul, Turkey 3Pathology, Private Practice, Istanbul,Turkey 4Department of Pediatric Surgery, Istanbul University Istanbul School of Medicine, Turkey 5Department of Biochemistry, Istanbul University Istanbul School of Medicine, Turkey
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Aim
To examine the effectiveness of a neutrophil elastase inhibitor, Sivelestat on ameliorating ischemia reperfusion injury in a muscle flap model.
Materials and Method
Eighteen male, 8-week old, specific pathogen-free Sprague Dawley rats were examined in 3 groups; Saline-treated control group(CG)(n=6), Ischemia group (IG)(n=6), Sivelestat-treated group(SG)(n=6). Gastrocnemius muscle flap was approached as previously described in studies. The sural artery branching from the politeal artery was clamped with 2v microvascular clamps. In the control group, the clamps were left in place for 5 hours of ischemia, prosecuted by 3 hours of reperfusion and saline was given during the reperfusion period.In the ischemia group, the gastrocnemius muscle flap was raised and islanded and the vascular pedicle was tied to mimic total ischemia. In Sivelestat group, the gastrocnemius muscle flap was raised as an islanded flap, the pedicle was clamped with 2v microvascular clapms for 5 hours, prosecuted by 3 hours of reperfusion. During the reperfusion period, a 16 gauge cannula was placed from the tail veins of the rats and Sivelestat was infused at a 10 mg/kg/hour dosage for 3 hours creating a pharmacological postconditioning procedure.After a total of 8 hours, the incisions were sutured and the rats in all groups were examined for 7 days in terms of wound healing, allergic reactions and general conditions. On the seventh day of reperfusion, gastrocnemius muscles were harvested for histological analysis with hematoxylene eozine and for myeloperoxidase activity and formeasuring the amount ofbicinchoninic acid which are both predictors of neutrophil elastase accumulation in the tissues.
Results
Inflammatory reaction in groups 1 and 2 were predominated by lymphocytes, followed by eosinophil leukocytes and plasmocytes resulting in edema collection. In group 3, the sivelestat-treated rats, the number of neutrophil leukocytes are much less, the inflammatory reaction is attenuated with less edema collection in between the tissues.Median tissue myeloperoxidase levels were significantly elevated in control muscle(group1) 2.45 (1.32-4.28) units /g wet weight) compared with that harvested from ischemic 1.12 (1.08-2.02) (p<0.001).
Conclusion
Use of Sivelestat will aid in decreasing the degree of myozite damage in upper limb replantations, crush syndromes and compartment syndromes and will help obtain more functional results in hand surgery where warm ischemia time is of utmost importance.
Keywords
Neutrophil elastase inhibition, ischemia reperfusion injury, Sivelestat, limb replantation
Introduction
Clinical situations associated with skeletal muscle ischemia-reperfusion injury (IRI)are commonly encountered in plastic surgery practice. Among these are compartment syndromes, crush injuries and vascular injuries including major and minor limb amputations as well as free flap surgeries in reconstructive procedures. When a tissue is reperfused following a period of ischemia, onemay experience an intense inflammatory response locally, as well as systemic effects including physiological, biochemical and immunological changes due to reactive oxygen species and activated neutrophils [1] and sometimes this may lead to a no-reflow phenomenon. Numerous factors have been implicated in the no-reflow phenomenon including edematous and retracting endothelial cells, intravascular fluid loss and microthrombus production[2]. This intense chain reaction may compromise the vascular circulation of the tissues and muscle tissue is the most prone tissue to ischemia reperfusion injury. Once the muscle is affected, the chances of a successful major limb replantation or the relief of a compartment syndrome diminishes. A replantation can be defined as successful, not only when the vascular circulation to the amputated digit or extremity is reestablished by microsurgery, but also when the functions of the digits are restored. Due to high metabolic demand of skeletal muscle, irreversable damage may ocur when reperfusion occurs even after brief ischemic intervals, this is especially relevant for compartment syndrome of the extremities. Several xanthine oxidase and free radical scavangers are proposed to be useful against the muscle IRI induced by oxidative stres damage[3, 4]. The role of neutrophil- elastase inhibitors in preventing IRI are more recently encountered[5]. Neutrophil elastase inhibitors reduces the levels of inflammatory mediators by inhibiting NF-κβ. Elastase is stored in cytoplasmic granules, its’ primary role being the intracellular degradation of foreign proteins during phagocytosis. The release of elastase during postischemic reperfusion may be an important mechanism by which neutrophils cause tissue injury. Sivelestat sodium hydrate is a synthetic specific low-molecular weight neutrophil elastase inhibitor(Ono Pharmaceutica Co, Osaka, Japan)[6].The drug competitively inhibits the neutrophil-elastase, but it does not affect other proteases such as plasmin, thrombin, kallikrein, cathepsin B or collagenase Ι2. Sivelestat has found use in acute systemic inflammatory response syndromes, acute respiratory distress syndrome and in acute lung injury clinically and ischemia reperfusion injury in the rat bladder experimentally[7].There is growing evidence regarding its’ beneficial effects in ameliorating ischemia-reperfusion injury in crush syndromes[8]. However, its’ role in reducing the damage in muscle tissue after IRI has not been addressed yet. In this experimental study, we aimed to examine the effectiveness of pharmacological postconditioning with Sivelestat, on muscle flap ischemia reperfusion injury model by histopathology and by quantitatively measuring the myeloperoxidase activity, which is a marker of neutrophil accumulation in the tissues.
Materials and Method
Animals all animal experiments were performed in accordance with the guidelines set by Bagcilar Research and Training Hospital local animal experimentation ethics committee and the experiments proceeded after obtaining an ethics approval from the same hospital. Eighteen male, 8-week old, specific pathogen-free Sprague Dawley rats, weighing 300 to 350 g, were purchased from Bagcilar Research and Training Hospital, Turkey. The experiments were performed at the same hospital. The animals were allowed to acclimatize for several days before use. Rats were housed in the laboratory animals center at the same hospital under standard temperature and light and dark cycles. Rats had access to chow and water ad libitum throughout the study. IRI on a muscle flap was created on a gastrocnemius muscle flap model, as previously employed by Akcal et al. [9]. The animals were divided into 3 groups(figure 1):
1) Saline-treated control group(CG)(n=6)2) Ischemia group (IG)(n=6)3) Sivelestat-treated group(SG)(n=6)
The rats were anesthetized with ketamine hydrochloride(40 mg/kg, Ketalar®) and xylazine(10 mg/kg, Rompun®) intraperitoneally and kept spontaneously breathing in room air. The animals were positioned prone and gastrocnemius muscle flap was approached as previously described in studies. The sural artery branching from the politeal artery was clamped with 2v microvascular clamps. In the saline treated control group, the clamps were left in place for 5 hours of ischemia, prosecuted by 3 hours of reperfusion where saline was administered through the tail vein. And following 3 hours of reperfusion, the incision was sutured with 3/0 polypropylene. In the ischemia group, the gastrocnemius muscle flap was raised and islanded and the vascular pedicle was tied with 3/0 silk suture in order to mimic total ischemia. In Sivelestat group, the gastrocnemius muscle flap was raised as an islanded flap, the pedicle was clamped with 2v microvascular clapms for 5 hours, prosecuted by 3 hours of reperfusion. During the reperfusion period, a 16 gauge cannula was placed from the tail veins of the rats and Sivelestat was infused at a 10 mg/kg/hour dosage for 3 hours creating a pharmacological postconditioning procedure. After a total of 8 hours, the incisions were sutured and the rats in all groups were examined for 7 days in terms of wound healing, allergic reactions and general conditions. On the seventh day of reperfusion, the rats were anesthetized once more with 40 mg/kg ketamin hydrochloride and 10 mg/kg xylazine and gastrocnemius muscles were taken as a biopsy detached from the achilles tendons. The gastrocnemius muscle flap was weighed with a microscale and observed for signs of inflammation and necrosis macroscopically.
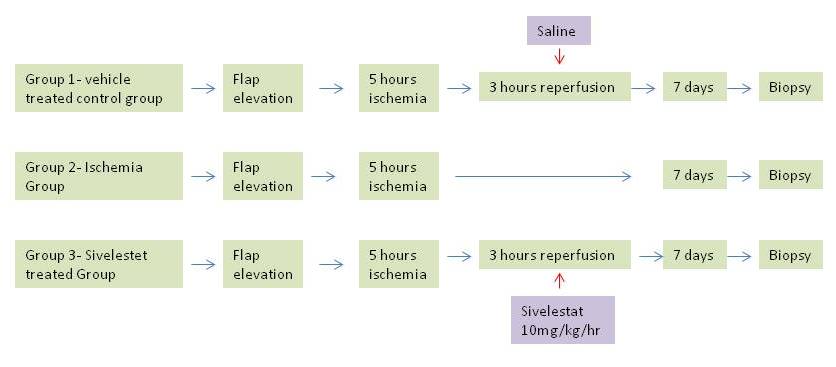
Figure 1: Protocol for induction of ischemia reperfusion injury protection in muscle flap
Histological evaluation
For a histological evaluation of each group, gastrocnemius muscle biopsies were retrieved transversely from the thickest portion of the muscle, fixed with 10% neutral formalin solution and embedded in paraffin. After that 6 µm- thick tissues were prepared by the senior pathologist and each tissue was stained with hematoxylin-eosin. The slides were analyzed in terms of myosite injury, vascular proliferation, oedema, acute and chronic inflammation. For quantitative analysis of neutrophil-elastase inhibition, myeloperoxidase immunassay analysis (MPO) was performed. The tissue samples of gastrocnemius muscle flap harvested from the study limbs were measured in control(n=6), ischemic(n=6) and sivelestat treated animals (n=6). Tissue myeloperoxidase (MPO) activities were measured according to the method described by Xia Y and Zweier JL [10]. 200 mg of muscle samples were homogenized in 3 mL 0.05 M K-phosphate buffer (pH: 6.0) containing 0.5% Hexadecyltrimethyl ammoniumbromide. Homogenates were then sonicated for 20 s and freeze-thawed 3 times. Suspensions were centrifuged at 40,000 xg for 15 min. 0.1 mL supernatant was mixed with 2.9 mL 0.05 M K-phosphate buffer (pH: 6.0) containing 0.53 mM O-dianisidine and 0.15 mM H2O2 and the change in absorbance at 460 nm was recorded for 5 min. Results are expressed as U/g wet tissue and nmoL/min/mg protein by a method described by Smith et al. [11] by measuring the amount ofbicinchoninic acid.
Data Analysis
The results are expressed in the form of mean ± SD. Serially measured parameters were compared with two-way analysis of variance (ANOVA). If the difference was statistically significant, an unpaired t test was used. To analyze the difference within each group, a repeated-measure ANOVA and a post hoc Bonferroni test were used. Other data between the two groups were compared with an unpaired t test. A p value less than 0.05 was considered statistically significant.
Results
A total of 18 animals were included with the mean weight of 350±40 g. All treatments were well tolerated. Overall elevated state of acute and chronic inflammation was observed in the saline- treated control group( group 1). Upon histological evaluation of IRI in the pedicled muscle flap with hematoxylin-eosin, modified Verhofstad scale was utilized to differentiate the damaged myosites from the undamaged myosites. (0= no degeneration, 1= degenerated myosites <10% of specimen, 2= degenerated myosites 10%-30% of specimen, 4=degenerated myosites >50% of specimen) (Figure 2),(Figure 3) degenerative histological scoring).As expected degenerated myosite percentage, was the highest among group 2, ischemia group, however there was a statistically significant difference among the vehicle- treated control group(group 1) and sivelestat treated group (group 3), with degenerative myosite percentages, 30-50% in group 1 and 10% in group 3 respectively( p<0.001). Microscopic slides of group 1 rats revealed dense degeneration of myosites which was directly proportional with dense vascular proliferation, elevated state of oedema between the myosites and dense inflammatory cells. Inflammatory reaction in groups 1 and 2 were predominated by lymphocytes, followed by eosinophil leukocytes and plasmocytes resulting in edema collection. In group 3, the sivelestat-treated rats, the number of neutrophil leukocytes are much less, the inflammatory reaction is attenuated with less edema collection in between the tissues. (figure 3)
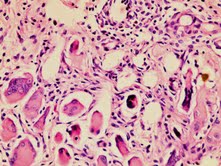
Figure 2: Ischemia reperfusion injury in group 1(H&E x100)
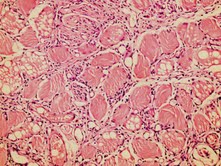
Figure 3: Ischemia reperfusion injury in group 3(H&E x100)
Muscle oedema is ameliorated by neutrophil elastase inhibition by sivelestat in our study and the differences were significant when compared with results for ischemic animals and saline treated control animals( p<0.001).
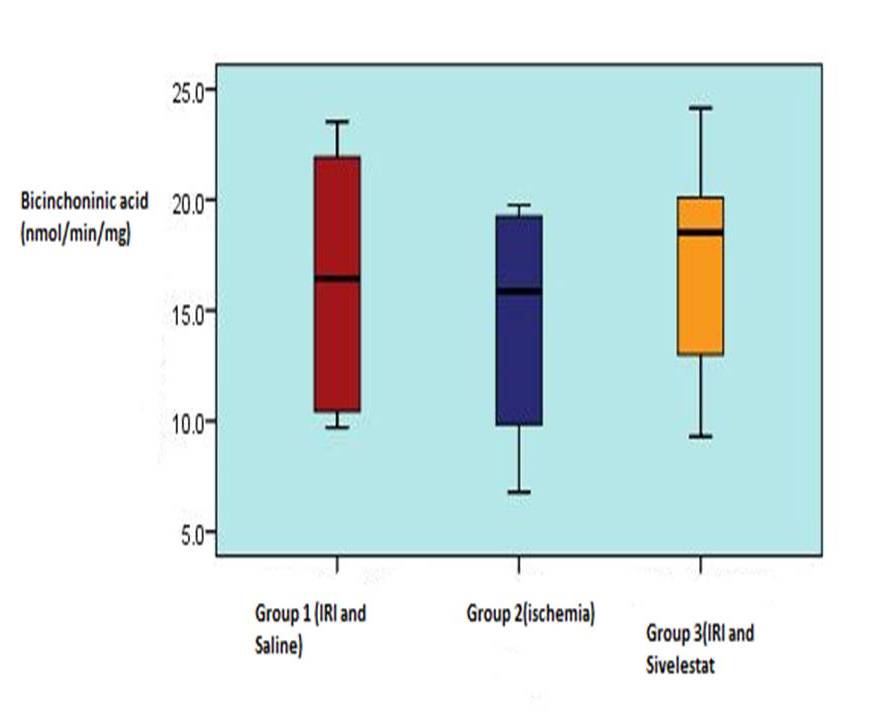
Table 12, 3, shows the muscle myeloperoxidase activity and bicinchoninic acid measurements in 3 experimental groups. Median tissue myeloperoxidase levels were significantly elevated in control muscle (group1) 2.45 (1.32-4.28) units /g wet weight) compared with that harvested from ischemic 1.12 (1.08-2.02) (p<0.001). This rise was significantly reduced in rats receiving Sivelestat 1.21(1.03-1.67) (p0.001) versus vehicle-treated control group.
|
Table 1 Measurementof
myeloperoxidase(MPO) activitiesin
muscletissueandmeasurement of bicinchoninicacid in muscletissue
|
|
|
Group 1(IRI andSaline)
mean±s.d
|
Group 2(ischemia)
mean±s.d
|
Group 3(IRI andSivelestat)
pmean±s.d
|
|
|
MPO activity(U/g)
|
1.38
±0.36
|
1.12
±0.41
|
1.21
±0.36
|
0.049
|
|
Bicinchoninicacid
(nmol/min/mg)
|
16.41
±5.79
|
14.56
±5.27
|
17.26
±5.30
|
0.068
|
|
ANOVA
|
|
IRI:
ischemiareperfusioninjury, s.d: standarddeviation
|
|
Discussion
Revascularization of skeletal muscle after acute prolonged ischemia may result in both local and systemic complications which are associated with significant patient morbidity and mortality [5, 12]. Such complications include the development of limb oedema, compartment syndrome, skeletal muscle necrosis, amputation, renal failure and pulmonary oedema. It has been well established that the major part of the injury occurs during reperfusion, rather than ischemia [13]. Macrophages are activated in ischemia reperfusion injuries and produce mediators including HMGB-1 and IL-8. The release of HMGB-1 and IL-8 would attract neutrophils to the injury site. Reactive oxygen species and activated neutrophls are the main factors responsible for local and systemic damage caused by IRI. Neutrophils contain several intracellular granules which embody myeloperoxidase, elastase and neutral proteases. Products of myeloperoxidase initiate lipid peroxidation in cell membranes [14, 15]. Following lipid peroxidation, cell membrane starts leaking. The sequestered neutrophils are activated as a result of interaction with endothelium, epithelium or interstitial matrix and trigger the uncontrolled release of proteolytic enzymes, including neutrophil elastase [16,17], while damaging the target tisuue, neutrophil elastase itself stimulates protease-activated receptor 2 and induces production of inflammatory cytokines such as IL-8, which leads to amplified sequesteration and activation of neutrophils and exacerbation of inflammation [17]. Sivelestat has been given on several trials to block this vicious cycle in ischemia reperfusion injury in rat bladder by Kono et al [7]. Takayama et al., reported that an alternative mechanism of sivelestat causing smooth muscle dilatation which is byinhibiting the substance-P induced contraction of guinea-pig airway smooth muscle by both epithelium dependent and independent mechanism [18]. Sivelestat has also been investigated on a crush injury model of 6 hours of crush injury and 3 hours of reperfusion and found to attenuate the muscle damage [8]. Although many studies have investigated the role of oxygen free radicals during ischemia-reperfusion, little consideration has been given to the potential of neutrophil derived proteases, of which neutrophil elastase is the most-abundant, to promote tissue damage. The present study has investigated whether pharmacologic postconditioning with Sivelestat is effective to reduce IRI in the muscle flap model by quantitative myeloperoxidase assay results indicate that neutrophil depletion prevented postischemic muscle necrosis. To the best of our knowledge, this is the first study assessing the influence of sivelestat in a gastrocnemius muscle flap model of IRI lesions [19]. Sivelestat showed a protective effect for muscle flap model and additionally the assessment of myeloperoxidase assay had robust precision as to the amount of neutrophil depletion. In our study, group 3, sivelestat treated group showed a lower myosite damage percentage and less chronic inflammation compared to the other groups. The concept of warm ischemia period becomes especially important in amputations, vascular injuries, crush syndromes andeven compartment syndromes [20,21]. The basic idea of applying sivelestat, a specific neutrophil-elastase inhibitor after ischemia has prevailed in a muscle tissue has been quite interesting for us which forms the basis of our study. Since sivelestat has initially found use in medicine in acute respiratory distress syndromes and acute pulmonary injuries, it has the advantage of blocking neutrophil- elastase systemically which is useful for the recovery of a person in a more gross scale. The success of major replantations of the upper limb also depends on less extensive muscle tissue loss which leads to a more functional extremity. We want to emphasize the potential benefits of post-conditioning either by pharmacological intervention( xanthine-oxidase inhibitors, oxygen –free radical scavangers), hyperbaric oxygen or hypothermia [22,23]. Our present data indicated that pharmacologic post-conditioning with sivelestat significantly decreased neutrophil production which is shown by measuring the amount of myeloperoxidase activity in the tissue. In conclusion, we want to propose this postconditioning regimen in major upper limb replantation surgery, compartment syndromes and crush syndromes since a neutrophil elastase inhibitor will help attenuate the vicious inflammatory cycle leading to remote organ injury as well as decreasing the amount of damaged myosites due to IRI of the muscle tissues.
Learning Objectives
1) Neutrophil elastase inhibitor Sivelestat ameliorates IRI in a muscle flap model2) Sivelestat has potential benefits if used as a postconditioning regimen3) Use of Sivelestat will aid in decreasing the degree of myozite damage in upper limb replantations, crush syndromes and compartment syndromes and will help obtain more functional results in hand surgery where warm ischemia time is of utmost importance.
Author’s Contributions
KZS: Designed the study , carried out the experiment, prepared the draftMY: Carried out the literature searchFAS: Performed the analysisMVK: Carried out the experimentAK: Carried out the literature searchSS:Carried out the experimentŞY: Performed the histological analysisDM: Prepared the draftBE: Performed the biochemistry analysis, interpreted the resultsAFA:Performed the biochemistry analysis
Conflict of Interest
None
Ethical Considerations
Bagcilar Animal Research Laboratory No: 212 12 February,2012
Funding
None
Refernces
[1]Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74: 1124-1136.[pubmed]
[2]Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon:2011.Plast Reconstr Surg. 2011; 685e.[pubmed]
[3]Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc Surg. 2002;10(6): 620-630.[pubmed]
[4]].Weiss APC, Carey LA, Randolph MA, Moore JR, Weiland AJ. Oxygen radical scavangers improve vascular patency and bone-muscle cell survival in an ischemic extremity replant model. Plast Reconstr Surg. 1989;84(1): 117-123.[pubmed]
[5]Crinnion JN, Homer-Vanniasinkam S, Parkin SM, Gough MJ. Role of neutrophil-endothelial adhesion in skeletal muscle reperfusion injury. Br J Surg.1996 ;83(2):251-254.[pubmed]
[6]].Fujimura N, Obara H, Suda K, Takeuchi H, Miyasho T, Kawasako K, Du W, Yamada S, Ono S, Matsumoto K, Yagi H, Kitago M, Shinoda M, Itano O, Tanabe M, Sakamoto M, Maruyama I, Kitagawa Y. Neutrophil elastase inhibitor improves survival rate after ischemia reperfusion injury caused by supravisceral aortic clamping in rats. J Surg Res. 2013;180(1):e31-36 .
[7]Kono T, Okada S, Saito M. Neutrophil elastase inhibitor, sivelestat sodium hydrate prevents ischemia-reperfusion injury in the rat bladder. Mol Cell Biochem. 2008;311:87-92.[pubmed]
[8]Cuong NT, Abe C, Binh NH, Hara A, Morita H, Ogura S. Sivelestat improves outcome of crush injury by inhibiting high-mobility group box 1 in rats. Shock. 2013;39(1):89-95.[pubmed]
[9]Akcal A, Sevim KZ, Yesilada A, Kiyak V, Sucu DO, Tatlidede HS, Sakiz D, Kaya H. Comparison of perivascular and intramuscular applied botulinum toxin a pretreatment on muscle flap ischemia-reperfusion injury and chemical delay. J Craniofac Surg. 2013;24(1):278-283.[pubmed]
[10]Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte-containing tissues Anal Biochem. 1997; 245: 93-96.[pubmed]
[11]Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al.Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85 [pubmed[
[12]Hosnuter M, Babuccu O, Kargi E, Altinyazar C. Dual Preconditioning: Effects of pharmacological plus ischemic preconditioning on skin flap survival. Ann Plast Surg. 2003;50:398-402.[pubmed]
[13]].Beyersdorf F, Unger A, Wildhirt A. Studies of reperfusion injury in skeletal muscle: preserved cellular viability after extended periodss of warm ischemia. J Cardiovasc Surg. 1991;32:664-676.[pubmed]
[14]Gillani S, Cao J, Suzuki T, Hak DJ. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43(6): 670-675.[pubmed]
[15]Bekerecioglu M, Kutluhan A, Demirtas I, Karaayvaz M. Prevention of adriamycin-induced skin necrosis with various free radical scavangers. J Surg Res. 1998;75:61-65.[pubmed]
[16]].Rubin BB, Smith Ai Liauw S, Isenman D, Romaschin AD, Walker PM. Complement activation and White cell sequestrationn in post-ischaemic skeletal muscle. Am J Physiol. 1990;259:H525-531.[pubmed]
[17]].Suda K, Takeuchi H, Hagiwara T, Miyasho T, Okamoto M, Kawasako K, Yamada S, Suganuma K, Wada N, Saikawa Y, Fukunaga K, Funakoshi Y, Hashimoto S, Yokota H, Maruyama I, Ishizaka A, Kitagawa Y. Neutrophil elastase inhibitor improves survival of rats with clinically relevant sepsis. Shock. 2010;33(5):526-531.[pubmed]
[18]Takayama N, Uchida K. Epithelium-dependent and -independent inhibitory effects of sivelestat, a neutrophil elastase inhibitor, on substance P-induced contraction of airway smooth muscle in lipopolysaccharide-treated guinea-pigs. J Smooth Muscle Res. 2005;41(5):257-270.[pubmed]
[19]Iwata K. The propensity score analysis on the efficacy of sivelestat. Pulm Pharmacol Ther. 2013;26(3):395.
[20]Urbaniak JR. Replantations. In: Green DP, editor. Operative Hand Surgery. Edinburgh:Churchill Livingstone;19993. P. 1085-1102.
[21]].Battiston B, Tos P, Clemente A, Pontini I. Actualities in big segments replantation surgery. J Plast Reconstr Aesthet Surg. 2007;60(7):849-855.[pubmed]
[22]Moon JG, Lim HC, Gye MR, Oh JS, Park JW. Postconditioning attenuates ischemia-reperfusion injury in rat skin flap. Microsurgery. 2008;28:531-537.[pubmed]
[23]Mowlavi A, Reynolds C, Neumeister MW, Wilhelmi BJ, Song YH, Naffziger R, Glatz FR, Russell RC. Age-related differences of neutrophil activation in a skeletal muscle ischemia-reperfusion model. Ann Plast Surg. 2003;50:403-411.[pubmed][pubmed]

