Original Article

Dexmedetomidine Pretreatment Attenuates Mesenteric Ischemia Reperfusion Injury in Rats
1A. Ebru Salman, 2Fahri Yetişir, 3İ. Özkan Önal, 4Dilara Zeybek, 3C. Öztuğ Önal, 5Banu Yürekli, 6İsmail Yürekli, 4Ayşegül Süzer, 7Mehmet Kiliç
- 1Department of Anesthesiology and Reanimation, Atatürk Research and Training Hospital, Ankara
- 2Department of General Surgery, Atatürk Research and Training Hospital, Ankara
- 3Etlik Research and Training Hospital
- 4Department of Histology and Embryology, Hacettepe University Faculty of Medine, Ankara
- 5Department of Endocrinology, Bozyaka Research and Training Hospital, Izmir
- 6Department of Cardiovascular Surgery, Atatürk Research and Training Hospital, Izmir
- 7Department of General Surgery, Assoc Prof. Yıldırım Beyazıt University, Ankara
- Submitted: February 13, 2013
- Accepted: February 22, 2013
- Published: February 22, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Aim
To investigate the effect of dexmedetomidine pretreatment in a rat model of mesenteric ischemia-reperfusion injury using biochemical markers and histopathological methods.
Material and Methods
A total of 28 female Sprague Dawley rats weighing between 230-300 gr were randomly divided in to 4 groups, 7 rats in each. Group I in which sham surgical preparation including isolation of SMA without occlusion was performed. Group II in which intestinal I/R was produced by clamping SMA for 1 hour and declamping for 3 hours, group III sham operated dexmedetomidine received dexmedetomidine at a dose of 25 mcg/kg i.p. Group IV dexmedetomidine was given at a dose of 25 mcg/kg i.p 30 min before intestinal ischemia induced. Rats were sacrificed at the end of the reperfusion period. Malondialdehyde (MDA), protein carbonyl (PC), superoxide dismutase (SOD), catalase, and glutathione peroxide (GPX) levels were analyzed in intestinal tissue samples. Tissue total antioxidant status (TAS), tissue total oxidant status (TOS), TNF alpha, IL6, IL10 values were measured from serum samples 3 hours after reperfusion. The histopathological examination scores were determined using the intestinal tissues.
Results
The mean TOS, TAS, GPx, SOD, catalase, MDA and IL10 values were not significantly different between group II and group IV. There were significant difference in the mean PC, TNF alpha and IL6 values between group II and group IV. The histopathological examination scores of intestinal tissues were significantly higher in group II compared to group IV (P<0.05).
Conclusion
Pretreatment with dexmedetomidine attenuates intestinal ischemia-reperfusion injury in rats. Dexmedetomine prevents remarkable morphological alterations in intestinal tissue and attenuates proinflammatory cytokines and protein oxidation.
Key Words
Dexmedetomidine, ischemia reperfusion, protein carbonyl, TNFα.
Introduction
Acute intestinal ischemia reperfusion (I/R) injury remains a devastating clinical phenomenon in medical and surgical emergencies requiring immediate intervention. Ischemic injury to intestine and subsequent reperfusion plays a fateful role in variety of clinical situations such as multiple trauma, shock and cardiovascular surgery [1]. Reperfusion of ischemic tissue has been shown to exacerbate acute ischemic injury through generation of inflammatory factors and release of reactive oxygen and nitrogen species [2]. Production of systemic inflammatory materials and cytokines initiates a cascade of events such as increased bowel permeability and bacterial translocation and multiple organ failure [2].
Several approaches have been used to reduce I/R injury of the intestinal mucosa and accelerate regeneration of mucosal function. Administration of propofol and other free radical scavengers, inhibition of NADPH oxides activity, prophylactic administration of L-arginin and glutamine have been applied with various successes in experimental studies [1, 3-5].
Dexmedetomidine a potent and highly selective alpha 2 adrenoreceptor agonist is used for sedation in ICU units [6]. It is also used as anesthetic adjuvant during surgery, provides good intraoperative hemodynamic stability [7]. It is used as a sedative and anesthetic adjuvant agent in the operating room and ICU because of its anti-inflammatory and cardio protective properties [8, 9]. Dexmedetomidine has demonstrated a protective effect against I/R injury of heart, kidney, testis and brain in several animal models either in vivo or in vitro [10-14].
The purpose of the present study was to investigate the effects of intraperitoneal single dose dexmedetomidine pretreatment on intestinal I/R injury in an in vivo rat model.
Material and Method
The current study was approved by Kobay D.H.L. A.Ş. Animal experiments local ethical committee and was performed in accordance with National Institutes of health guidelines for the use of experimental animals. Twenty eight adult pathogen free female Sprague Daley rats weighing 230-300 gr were housed in indivual cages in temperature controlled room with alternating 12 hr light/ dark cycles, acclimated for 1 week before study and all animals had free access to water.
The rats were randomly allocated in to one of the 4 groups. Group I in which sham surgical preparation including isolation of SMA without occlusion was performed. Group II in which intestinal I/R was produced by clamping SMA for 1 hour and decamping for 3 hours, group III sham operated dexmedetomidine, received dexmedetomidine at a dose of 25 mcg/kg i.p. Group IV in which dexmedetomidine was given at a dose of 25 mcg/kg i.p 30 min before intestinal ischemia induced. Drugs were dissolved in normal saline.
All animals were anesthetized with ketamine 40 mg/kg i.m and xzylazine 5 mg/kg i.m). The rats were allowed to breathe spontaneously. Superior mesenteric artery (SMA) was isolated near its aortic origin via midline laparotomy in all animals. Both this artery and collateral branches coming from the celiac axis and inferior mesenteric artery were occluded with atraumatic vascular clips for 60 minutes. The absence of arterial pulsation distal to the clip or pale color of small intestine confirmed adequate occlusion. Entire bowel was covered with sterile pads soaked in saline at 37 OC in order to lessen heat loss and evaporation. After the clip was opened, the abdominal incision was closed and followed by 3 hours reperfusion period. The return of pulses and recoloration of small intestine were assumed to indicate adequate reperfusion of intestine.
Rats were sacrificed at the end of the reperfusion period. Tissue samples were obtained from ileum and jejunum for histopathological examination. A segment of intestine 1 cm was cut from 5 cm to terminal ileum, a portion of intestine 1 cm; 30 cm distal to the ligament of Treitz was cut and washed with cold saline, fixed in formaldehyde polymerization (4%) and embedded in paraffin for preparation. Remaining part of the small intestine washed with cold saline, dried and weighed.
Histopathological examination: Intestinal samples from proximal jejunum and distal ileum were rapidly fixed in 10% phosphate-buffered formalin; then they were dehydrated through graded alcohols and processed for routine light microscopy. All specimens were embedded in paraffin wax. Deparaffinized sections (5µm) were stained with haematoxylin and eosin (H&E) according to standard protocol and photographed by using a light microscope (Leica DM6000B, Wetzlar, Germany) with a DC490 digital camera (Leica, Wetzlar, Germany).Villous height and crypt depth for each specimen were measured in 10 villi and 10 crypts from jejunum and ileum sections using Leica Application Suite image analysis software (Leica, Wetzlar, Germany). Injury in intestinal mucosal tissues in all groups was evaluated according to Chiu’s classification [15]. 0, Normal mucosal villi; 1, sub epithelial space at the tips of the villi; 2, Moderate elevation of the epithelial layer from lamina propria; 3, Massive epithelial elevation extending down sides of villi, a few tips may be denuded; 4, Denuded villi with lamina propria exposed and dilated capillaries; and 5, Disintegration of lamina propria; hemorrhage, and ulceration.
Biochemical Analysis: All tissues were washed 2 times with cold saline solution placed in to glass bottles, and stored in a deep freezer-80°c until processing. The intestinal tissues were homogenized in 10 volumes of 150 Mm ice-cold KCl using a glass Teflon homogenizer (Ultra Turrax IKA T18Basic, IKA Labortecnic,Staufen, Germany) for 2 min at 5000 rpm after cutting the tissues in to small pieces. The homogenate was then centrifuged at 4000 g for 15 min and the supernatant analyzed. MDA, catalase, sod and GPX and protein carbonyl were measured in tissue samples.
Two ml intracardiac blood was withdrawn 3 hours after reperfusion. The whole blood was centrifuged at 3500 rpm for 15 min at 4°C and plasma collected and stored at -80°C. Cytokines (TNFα, IL6, IL10), total tissue oxidant status (TOS) and total tissue antioxidant status (TAS) were measured at serum samples.
Measurement of cytokines: TNFα, IL6, IL10 levels were evaluated in plasma samples 3 hours after reperfusion. The assay was carried out by a colorimetric commercial kit. (Calbiochem-Novabiochem Corp,San Diego, CA, USA).
Measurement of Malondialdehyde: the lipid per oxidation product, intestinal tissues was homogenized in 1.15% KCl solution. An aliquot of (100 mcl) of the homogenate was added to a reaction mixture containing 200 mcl of 8.1% sodium dodoecyl sulphate 1500 mcl of 20% acetic acid (Ph 3.5), 1500 mcl of 0.8% thiobarbituric acid and 700 mcl distilled water. Samples were then boiled for 1 hr at 95 Oc and centrifuged at 3000 g for 10 min. The absorbance of supernatant was measured by spectrophotometry at 650 nm. The results were calculated as nmol.100 mg-1 tissue.
Measurement of superoxide dismutase: Superoxide dismutase activity was evaluated by inhibition of nitro blue tetrazolium reduction by superoxide anion generated by the xhanthine/xhantineoxide system using a commercial assay kit (Nnjing Jiancheng Biological Product, Nanjing China.) The results were expressed as U.100mg-1 protein.
Measurement of Catalase: Cayman’s catalase assay kit was used to determine the activity of catalase. The method is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of H2O2.The formaldehyde produced was measured calorimetrically in tissue homogenate.
Measurement of Glutathione peroxide: Cayman GPX assay kit was used to measure the activity of GPX. The rate of decrease in the A 340 is directly proportional to the GPX activity in tissue sample.
Measurement of Potein carbonyl: Cayman’s protein carbonyl colorimetric assay kit was used. The amount of protein hydrozone is quantified spectrophotometrically at an absorbance between 360-385 nm. The carbonyl content was standardized to protein concentration.
Measurement of TAS: It was performed using an aero set 2.0 analyzer and a total antioxidant status kit. The assay results are expressed as mmol Trolox equivalent/L.
Measrement of TOS:It was performed by using an Aeroset 2.0 analyzer and A TOS kit. Te results are expressed as the micro molar hydrogen peroxide equivalent per liter.
Statistical Analysis
The statistical analysis was performed by using the statistical package for the social sciences version 17, 0 for windows. (SPSS, Chicago, IL) The data are expressed as mean ± Standard Deviation. One way analysis of variance Anova test was used in statistical analysis of parameters, for comparison of the groups. Post-hoc Turkey test was used for secondary comparisons. p<0.05 was considered as statistically significant.
Result
The jejunum and the ileum exhibited normal mucosal morphology with intact simple columnar epithelium in Group I and Group III (Figure 1A, B, E, F). Treatment with dexmedetomidine did not cause any injury in the intestine compared to that in group I. The epithelial lining of villi was degenerated and desquamated to the lumen in both jejunum and ileum in Group II (Figure 1C, D). The mean intestinal injury grade of group II was significantly increased in jejunum and ileum compared to group I (p
<0.05) (Figure 2). The mean intestinal injury grades (Chiu’s scores) of both jejunum and ileum were significantly decreased in group IV when compared to group II (p<0.05) (Figure 1G, 1H).
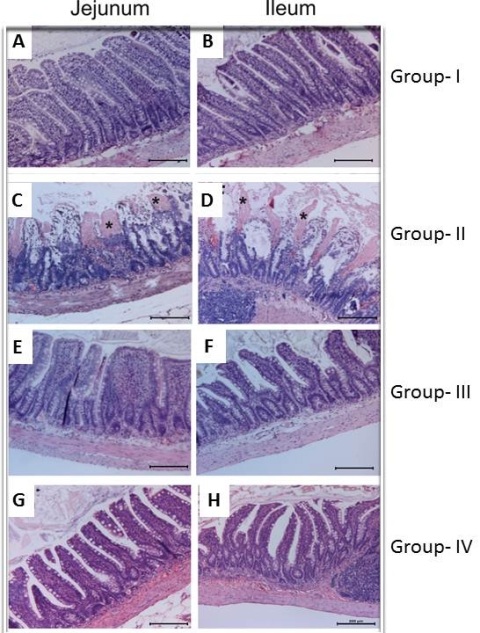
Figure 1: Effect of ischemia reperfusion and dex in jejunum and ileum. (Stained with hematoxylin-eosin, magnification X100) Normal intestinal histology of jejunum and ileum in both group 1 (control) and group 3 (DEX) (A, B, E,F). Massive epithelial sloughing and denuded villi (asteriks) in both jejunum and ileum of IR group (C, D). Most of the villi were covered by epithelium at the tips in both jejunum and ileum of dext pretreated IR group (G, H).
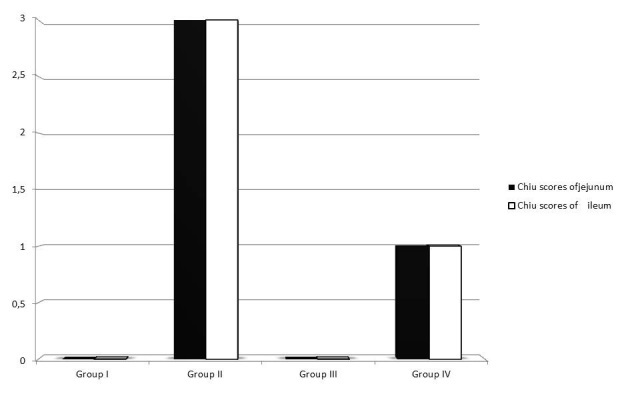
Figure 2: The changes in Chiu’s scores in intestinal tissue in groups.
There was no statistically significant difference in the villi height and crypt depth in between group I and group III. Lower villus height in jejunum (216.54 ± 5.77 vs. 304.39 ± 7.33 µm, p<0.008) and ileum (216.10 ± 5.19 vs. 287.89± 8.29 µm, p<0.004) as well as crypt depth in jejunum (108.55 ± 2.96 vs. 136.11 ± 2.16 µm) (p=0.246) and ileum (107.35 ± 2.15 vs. 130.39 ± 1.94 µm p<0.017) was detected in group II compared to group I. Villus height in jejunum (209.051 ± 6.85 vs. 216.54 ± 5.77 p<0.996) and ileum (213.17 ± 7.66 vs. 216.10 ± 5.19 µm p<0.707) was not different between group IV and group II. The depth of crypts was also increased in jejunum (119.08 ± 3.19 vs. 108.55±2.96 µm) (p= 0,021) and ileum (119.98 ± 2.44 vs 107.35± 2.15 µm p<0.046) in group IV compared to group II (Figure 3).
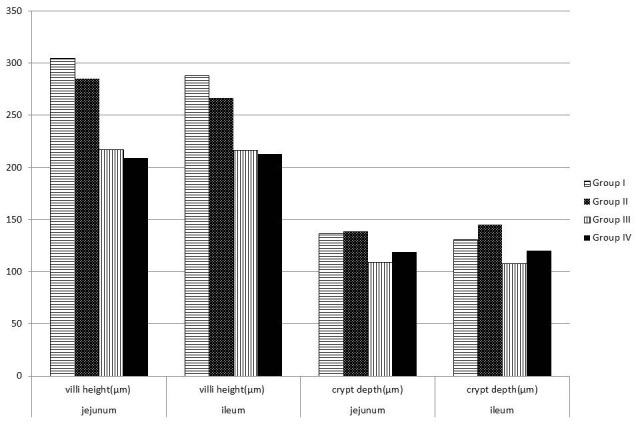
Figure 3: The changes in the villus heights and crypt depths in ileum and jejunum in groups.
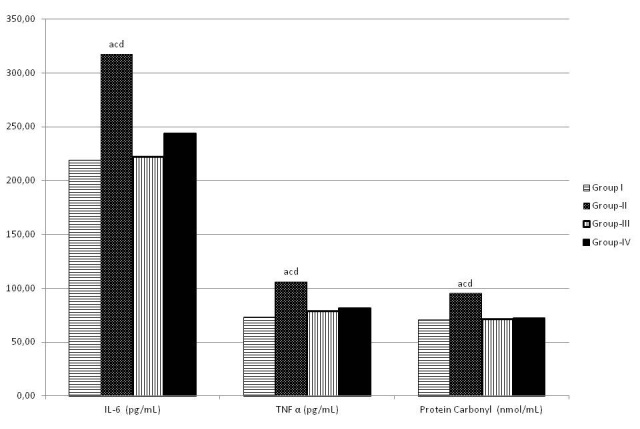
Figure 4: The changes in IL 6, TNFα and PC levels in groups.
Reperfusion of ischemic splanchnic circulation led to a substantional increase in TNFα and IL6 levels in group II and group IV (Figure 4). Pretreatment with dexmedetomidine attenuated the increase in TNFα (P=0.012) and IL 6 (p=0.001) levels significantly (Table 1). TAS and TOS levels were similar between groups. (Table 1) IL 10 level was not different between group II and group IV (Table 1). Protein carbonyl level which is an indicator of protein oxidation was significantly higher in group IV compared to group II (Table 2). There is significant difference in levels of lipid per oxidation product MDA in group II compared to group I (p<0.001), no difference between group IV and group II (Table 2). There was no significant difference in SOD, catalase and GPX between group II and group IV (Table 2).
Table 1: The levels of TOS, TAS, TNFα, IL 6 and IL 10 in groups
|
Group-I |
Group-II |
Group-III |
Group-IV |
| TOS (mmol/L) |
2,15±0,7 |
1,86±1,22 |
1,29±0,49 |
1,58±1,14 |
| TAS (umol/L) |
0,86±0,38 |
0,86±0,7 |
0,29±0,49 |
0,86±0,9 |
| TNFα (pg/mL) |
72,58±11,71α |
105,43±11,48*π© |
78±15,48α |
80,72±15,5α |
| IL-10 (pg/dL) |
47,72±6,13 |
61,72±17,3 |
45,43±3,11α |
71±26,93 |
| IL-6 (pg/mL) |
218,58±35,25α |
317±34,52*π© |
222±25,1α |
243,43±46,39α |
* p<0.05 vs the group I αp<0.05 vs the group II πP<0.05 vs the group III ©P<0.05 vs the group IV
Discussion
Acute mesenteric ischemia is a devastating clinical entity with a high mortality rate, even with a successful diagnosis and medical and surgical therapies. I/R injury in small intestine may develop during septic shock, hemorrhagic shock, abdominal aortic artery surgery, coroner artery bypass grafting [1, 16-19]. Sensitivity of intestinal mucosa to I/R injury, and relative incapability of increasing oxygen transport in hypoxic stress predisposes the gut to subsequent necrosis. Restoration of blood flow and reintroduction of oxygen to ischemic intestine may lead to more severe functional and morphological changes than the injury produced by ischemia itself [20]. Endothelial dysfunction which is an important component of the exacerbation of the shock state, predisposes to vasospasm, platelet aggregation, and increased neutrophil adherence [21]. Reduced endothelial derived nitric oxide (NO), which is one of the earliest manifestations of I/R injury leads to increased leucocytes-endothelial interaction [21, 22]. Schleiffer et al., demonstrated that L-arginin and molsidomine which are the exogenous sources of NO given entirely before ischemia increased survival and improved intestinal mucosal barrier function [4].
Table 2: The levels of SOD, MDA, catalase, GPX and PC in groups
|
Group-I |
Group-II |
Group-III |
Group-IV |
| SOD (U/mL) |
1,03±0,14α |
0,75±0,18*π |
0,98±,10α |
0,94±013 |
| Catalase activity (nmol/min/mL) |
130,58±16,72 |
120,29±12,36 |
125,29±8 |
125,15±15,52 |
| MDA (ng/ml) |
3,29±0,03α© |
3,50±0,03*π |
3,30±0,06α© |
3,45±0,09*α |
| GPx (nmol/min/ml) |
40,15±9,34 |
33,15±11,4 |
41±7,6 |
36,29±31,9 |
| Protein Carbonyl (nmol/mL) |
71,68 ±13,34α |
94,90 ± 16,88*π© |
72,30 ±90,10α |
74,56 ± 15,32α |
*p<0.05 vs. the group l αp <0.05 vs. the group II πp<0.05 vs. the group III ©p<0.05 vs. the group IV
Alpha 2 adrenergic receptor agonists are useful and safe adjuncts in diverse clinical applications for their sedative, analgesic, preoperative sympatholytic, anesthetic sparing and hemodynamic stabilizing and potential neuroprotective properties [23]. Dexmedetomidine, a highly selective alpha 2-adrenergic receptor agonist with a relatively high ratio of alpha2/alpha 1 activity (1620:1 as compared to 220:1 for clonidine) offers a unigue ability to provide conscious sedation and analgesia without respiratory depression [6].
Some chemicals and oxygen radical scavengers such as resveratrol, ascorbic acid, melatonin and L-carnitine has been used to attenuate mesenteric I/R injury in animal models [24]. But serious side effects and clinical unapplicability are the pitfalls for these substances.
Vasilleiou concluded that use of propofol prevents intestinal I/R injury induced lung injury [25]. The effects of ketamine were investigated and were shown that ketamine protects intestine against I/R injury [26]. Therefore, the effects of anesthetic and sedative agents already used in anesthesia become a noteworthy point in reducing injury related to intestinal I/R.
It was shown that pretreatment with dexmedetomidine prevented the histopathological disruption significantly. Our study does not point out whether or not impaired structure was associated with altered function.
The result of our study demonstrated that dexmedetomidine pretreatment at a dose of 25 mcg/kg i.p attenuated the level of TNF alpha and IL 6. This finding suggested that dexmedetomidine might confer its intestinal protection by inhibiting inflammatory response [27-29]. The same reduction in TNF alpha and IL 6 was demonstrated in previous studies as well [24]. It was also shown in our study that protein carbonyl was significantly lower in dexmedetomidine pretreated ischemia group suggesting that dexmedetomidine might show its protective effect against intestinal I/R injury by attenuating protein oxidation.
Dexmedetomidine did not attenuate the MDA level in concordance with the previous studies, in which it was speculated that either the dose used in this study was not adequate to prevent lipid per oxidation or oxidative stress and neutrophil accumulation could not be involved in the mechanism of dexmedetomidine for protection against intestinal ischemia reperfusion [10].
The mechanism of the dexmedetomidine against intestinal ischemia reperfusion is not clearly identified. It was proposed that inhibition of ischemia-induced noradrenalin secretion from the presynaptic alpha adrenoreceptors, could prevent the destructive effects of I/R injury [30]. Decrease in the level of the inducible nitric oxide syntheses activity could be another possible explanation for this protective effect [24]. Schaak et al., showed that the activation of alpha 2 adrenoreceptor increased intestinal epithelial cell proliferation [31]. Apoptosis is the major form of cell death in the destruction of rat intestinal epithelial cells, the main component of the intestinal mucosal barrier. It has been demonstrated that dexmedetomidine has antiapototic effects by inhibiting intrinsic apoptotic cascade either via reducing proapoptotic protein bax expiration and increasing the antiapototic bcl2 expression [10]. Antiapopotic effect of dexmedetomidime might be associated with inhibition of the activation of extrinsic cascade by reducing the production of TNFα [32].
We chose an acute and near complete ischemia model by occluding SMA with interruption of collaterals in this experimental study. The dose of dexmedetomidine used in this study was determined based on a previous study [33]. Dexmedetomidine mainly absorbed into blood circulation after i.p injection of 25mcg/kg could have produced a substantially larger concentration in the intestinal mucosa than would have resulted from a smaller dose of dexmedetomidine administered intravenously. It was demonstrated that early moments of reperfusion are critical for intestinal protection [10]. Zhang et al., showed that early intervention of dexmedetomidine is critical for intestinal protection, hence we applied dexmedetomidine 30 min before ischemic insult. For clinical benefit for the patients with potential intestinal ischemia, dexmedetomidine should be used as before surgery.
There were several limitations of our study. We used only single dose of dexmedetomidine i.p to study intestinal protection offered by dexmedetomidine. Further investigations are warranted to study the dose-effect relationship and clarify the exact mechanism of this effect. Pretreatment of appropriate dose of dexmedetomidine may provide a new insight in critical clinical setting related to intestinal I/R injury and to define a perioperative algorhythm for patients with potential intestinal ischemia. Anesthetic agents may be chosen not only depending on their anesthetic and sedative properties but also their probable pleiotrophic effects to attenuate the deleterious effects of intestinal I/R injury.
Author’s Contribution
AES: Planning, collecting data, writing.
FY: Planning, collecting data, statistical analysis.
IOO: Collection of data.
DZ: Histological examination.
COO: Planning of manuscript.
BY, IY: Writing and evaluation of data.
AS: Histological examination.
MK: Planning, Evaluation of data.
Conflicts of Interest
None
Ethical Considerations
Kobay Animal Research Labaratory.No:29-22.11.2011.
Funding
The author declares Etlik Research and Trainning Hospital Research Funding.
Acknowledgement
None
References
[1]. Demirkan A, Orazakunov E, Savasş B, Kuzu AM, Melli M. Enteral glutamine pretreatment does not decrease plasma endotoxin level induced by ischemia-reperfusion injury in rats. World J of Gastroenterol 2008;14:463-8. [Pubmed].
[2]. Sukhotnik I, Helou H, Lurie M, Khateeb K, Bejar J, Coran A.G et al.The effect of leptin on intestinal recovery following ischemia-reperfusion injury in a rat. Pediatr Surg Int.2007;23:473-78. [Pubmed].
[3]. Liu KX, Rinne T, He W, Wang F,Xia Z. Propofol attenuates intestinal mucosa injury induced by intestinal ischemia–reperfusion in the rat. Can J Anesth 2007;54:366-74. [Pubmed].
[4]. Schieffler R, Raul F. Prophylactic administration of L arginine improves the intestinal barrier function after mesenteric ischemia. Gut 1996;39:194-8. [Pubmed].
[5]. Paterniti I, Galuppo M, Mazzon E, Impellizzeri D, Esposito E, Bramanti M et al. Protective effects of apocynin, an inhibitor of NADPH oxidase activity in splanchnic artery occlusion and reperfusion. JLB 2010:88:993-1003. [Pubmed].
[6]. Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opinion in Anesthesiol 2008;21:457-61. [Pubmed].
[7]. Tanskanen PE, Kytta JV, Randell TT, Aantaa RE. Dexmedetomidine as an anesthetic adjuvant in patients undergoing intracranial tumour surgery: A double blind randomized and placebo controlled study. Br J Anaesth 2006;97:658-65. [Pubmed].
[8]. Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function and cardiovascular endocrine and inflammatory responses in postoperative patients needing sedation in intensive care unit.Br J Anaesth 2001;86:650-6. [Pubmed].
[9]. Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications: A meta analysis. Am J Med.2003;114:742-52. [Pubmed].
[10]. Zhang XY, Liu ZM, Wen SH, Li YS, Yao X, Huang WQ, et al. Dexmedetomidine administration before but not after ischemia attenuates intestinal injury induced by intestinal ischemia reperfusion in rats. Anesthesiology 2012; 116:1035-46. [Pubmed].
[11]. Okada H, Kurita T, Mochizuki T, Morita K, Sato S. The cardioprotective effect of dexmedetomidine on global ischemia in isolated rat hearts. Resuscitation.2007;74.538-45. [Pubmed].
[12]. Koçoğlu H, Öztürk H, Öztürk H, Yılmaz F, Gülcü N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney. A histopathologic study. Ren Fail 2009;31:70-4. [Pubmed].
[13]. Engelhard K, Werner C, Eberspacher E, Bchl M, Blobner M, Hildt E, Hutzler P, Kochs E. The effect of alpha 2 agonist dexmedetomidine and the N-methyl D-aspartate antagonist S (+) ketamine on the expression of apoptosis regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg 2003;96:524-31. [Pubmed].
[14]. Hancı V, Erol B, Bektaş S, Mungan G, Yurtlu S, Tokgöz H, Can M, Ozkoçak Turan I. Effect of dexmedetomidine on testicular torsion/detorsion damage in rats. Urol Int 2010;84:105-11. [Pubmed].
[15]. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states: I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970; 101: 478-83. [Pubmed].
[16]. Tadros T, Traber DL, Heggers JP, Herndon DN. Effects of interleukin-1 administration on intestinal ischemia and reperfusion injury, mucosal impermeability and bacterial translocation in burn and sepsis. Ann Surg 2003;237:101-9. [Pubmed].
[17]. Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, et al. Functional and morphological changes of the gut barrier during the restution process after hemorrhagic shock. World J Gastroenterol.2005;11:5485-91. [Pubmed].
[18]. Juel IS, Solligard E, Lyng O, Stromholm T, Tvedt KE, Jonhsen H et al. Intestinal injury after thoracic aortic cross-clamping in the pig.J Surg Res 2004;117:283-95. [Pubmed].
[19]. Bjorck M, Troeng T, Bergqvist D. Risk factors for intestinal ischemia after aortailiac surgery:a combined cohort and case control study of 2824 operations. Eur J Endovasc Surg 1997;13:531-9. [Pubmed].
[20]. Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Phsiol 1986;250:G749-G753. [Pubmed].
[21]. Cuzzocrea S, Misko TP, Costantino G, Mazzon E, Micali A,Caputi AP et al. Beneficial effects of peroxynitrite decomposition catalyst in a rat model of splanchnic artery occlusion and reperfusion. FASEB J 2000;14:1061-72. [Pubmed].
[22]. Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitricoxide release after myocardial ischemia reperfusion promotes neutrophill adherence to coronary endothelium. Circ Res. 1993; 72:403-12. [Pubmed].
[23]. Grewal A. Dexmedetomidine : New Avenues. J Anesthesiol Clin Pharmacol 2011;27:297-302. [Pubmed].
[24]. Kılıç K, Hancı V, Selek Ş, Sözmen M, Kılıç N, Çitil M et al. The effects of dexmedetomidine on mesenteric arterial occlusion-associated gut ischemia and reperfusion induced gut and kidney injury in rabbits. J Surg Res. 2012;apr doi:10.1016/j.jss.2012.03.073. [Pubmed].
[25]. Vasileiou I, Kalimeris K, Nomikos T, Xanthopoulou MN, Perrea D, Agrogiannis G et al. Propofol prevents lung injury following intestinal ischemia-reperfusion. J Surg Res 2012;172:146-52. [Pubmed].
[26]. Camara CR, Guzman FJ, Barrera EA, Cabello AJ, Garcia A, Fernandez NE et al. Ketamine anesthesia reduces intestinal ischemia reperfusion injury in rats .World J Gastroenterol 2008 ;14 :5192-6. [Pubmed].
[27]. Ikeda H, Suzuki y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G. Apoptosis is a major mode of cell death caused by ischemia and ischemia/reperfusion injury to the rat intestinal epithelium. Gut 1998;42:530-7. [Pubmed].
[28]. Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Phsiol. 1998;274:G270-6. [Pubmed].
[29]. Mondello S, Galuppo M, Mazzon E, Domenico I, Mondello P, Carmela A, Cuzzocrea S. Glutamine treatment attenuates the development of ischemia/reperfusion injury of the gut. Eur J Pharmacol 2010;643:304-15. [Pubmed].
[30]. Jolkkonen J, Puurunen K, Koistinaho J, Koistinaho J, Kauppinen R, Haapalinna A, et al. Neuroprotection by the alpha 2 adrenoreceptor agonist dexmedetomidine in rat cerebral focal ischemia. Eur J Pharmacol 1999;372:31-6. [Pubmed].
[31]. Schaak S, Cussac D, Cayla C, Devedjian JC, Guyot R,Paris H et al. Alpha 2 adrenoreceptors regulate proliferation of human intestinal epithelial cells. Gut 2000:47:242-50. [Pubmed].
[32]. Diebel LN, Liberati DM, Baylor AE 3rd, Brown WJ, Diglio CA. The pivotal role of tumor necrosis factor alpha in signaling apoptosis in intestinal epithelial cells under shock conditions. J Trauma 2005,58:995-1001. [Pubmed].
[33]. Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemiareperfusion in mice. Acta anesthesiol scand 2011;55:1272-8. [Pubmed].

