3Department of Obstetrics and Gynecology, Institute of medical science, Banaras Hindu University, India
- Submitted: May 05, 2013;
- Accepted: July 26, 2013;
- Published: July 26, 2013
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Primary ovarian leiomyoma is a very rare benign tumour that usually represents an incidental finding at surgery or autopsy. We report a case of 35-year-old hysterectomized lady presenting with a large cystic abdominal lump which was clinically diagnosed as a malignant ovarian mass but proved to be degenerated leiomyoma on histopathology. We briefly discuss rarity and pathogenesis of this case.
Key-words
Leiomyoma, ovary, degeneration, smooth muscle, immunohistochemistry
Introduction
Leiomyoma is a benign smooth muscle tumour that most commonly affects the uterus, cervix and broad ligaments in women of reproductive age group (1). Primary leiomyoma of the ovary is a very rare benign tumour as only about 70 cases have been described until 2004 (2). It usually represents an incidental finding on routine pelvic examination, at surgery or autopsy (3). We herein report an interesting case of a massively enlarged ovarian leiomyoma with degeneration that simulated ovarian malignancy in a middle-aged lady and discuss its pathogenesis in brief.
CasePresentatin
A 35-year-old (para 4, gravida 0) hysterectomized lady presented with a complaint of swelling in the abdomen since one month. Her abdominal examination revealed an ill-defined cystic lump of around 28-30 weeks uterine size, arising from pelvic cavity. The vault was normal. A computed tomography (CT) scan showed a large complex relatively well-delineated cystic mass of 25.1C24.5C12.1 cm size with internal septations (Fig.1A).
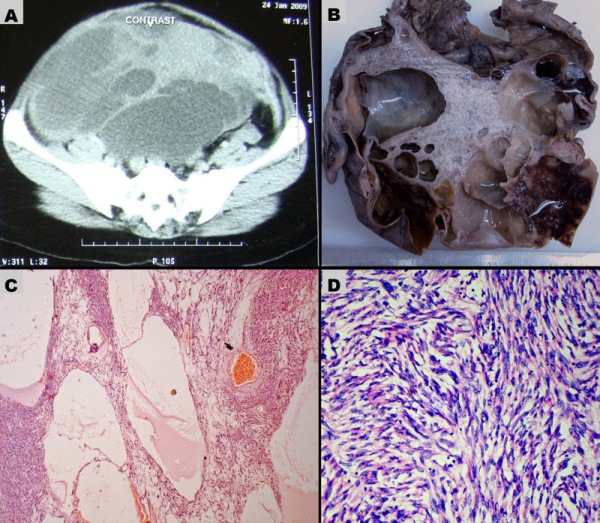
Figure 1: (A) Pelvic CT scan shows left ovarian cystic mass with multiple internal septations and adjacent solid areas. The mass is completely occupying the pelvic cavity. (B) Cut surface of the ovarian mass shows multiloculated cysts and adjacent grey white solid area. Few cysts contain blood clots. (C-D) Microscopic sections of the ovarian leiomyoma. (C) Pseudocystic spaces contain edema fluid and surrounding cellular areas of spindle cells (H&E, 100X). (D) Cellular area shows criss-cross fascicles of spindle cells, a feature of leiomyoma (H&E, 400X).
The mass was occupying the pelvic and abdominal cavities and did not show infiltration to the adjoining structures. The CT scan findings suggested a malignant left ovarian mass. The routine laboratory investigations were within normal limit. Carbohydrate antigen-125 (CA-125) level was 28.9 U/ml (Normal <14.7 U/ml). A laparotomy revealed a large cystic left ovarian mass with characteristics as noted in the CT scan. The omentum was found adhered to anterior surface of the mass. The mass was excised and omentectomy was done. The resected ovarian mass, salpinx and omentum were fixed in 10% formalin solution and submitted for histopathological examination./p>
The specimen received was an ovarian mass measuring 24C20C13 cm with attached fallopian tube. The external surface was bosselated and congested. Cut surfaces showed multiloculated cysts containing haemorrhage and blood clots and a small solid part that appeared whorled and myxoid (Fig.1B). Haematoxylin and eosin (H&E) - stained sections of the tumour tissue showed multiple pseudocystic spaces, surrounded by spindle cells of variable cellularity (Fig. 1C). The pseudocystic spaces lacked a true epithelial lining and contained edema fluid and red cells in the lumina. In highly cellular areas, spindle cells were arranged in interlacing fascicles whereas this arrangement was lost in more edematous, degenerated and hypocellular areas. The spindle-shaped cells had regular, elongated, blunt- ended nuclei and eosinophilic cytoplasm, resembling leiomyoma (Fig.1D). Significant nuclear atypia, nuclear pleomorphism and mitotic activity were absent. Considerable part of the tumour showed degenerative changes viz., cystic degeneration, myxoid degeneration with multiple foci of hemorrhage, thrombosis and necrosis. Multiple sections examined from the mass did not show remnant ovarian tissue. Omental fat and attached fallopian tube was unremarkable on histology. Immunohistochemistry performed on sections from tumour showed moderate-to-strong diffuse cytoplasmic positivity for desmin and smooth muscle actin (SMA) (Fig. 2A-B).
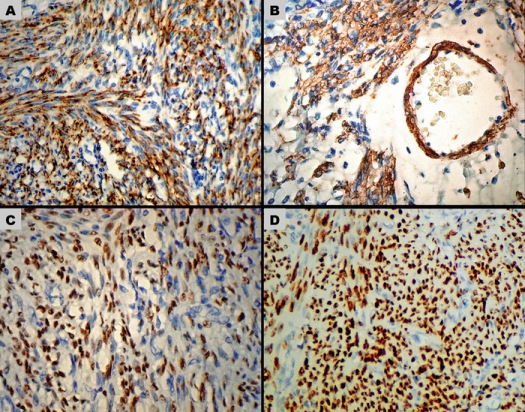
Figure 2: Immunohistochemical staining in the ovarian leiomyoma. (A) Diffuse strong cytoplasmic positivity for Desmin (400X). (B) Diffuse cytoplasmic staining for SMA (400X). The smooth-muscle cells in a blood vessel wall have also stained positive. (C) Positive ER staining of the leiomyoma cell nuclei (200X). (D) In comparison to (C), diffuse strong nuclear staining for PR is seen (200X).
The nuclei expressed positive staining for estrogen receptor (ER) and progesterone receptor (PR). In comparison to ER, nuclear staining of PR was diffuse and more intense (Fig. 2C-D). Overall, the findings were consistent with degenerated leiomyoma of ovary. Patient had an uneventful post-laparotomy recovery and she was discharged in a stable condition after one week.
Discussion:
Primary leiomyoma of the ovary is a very rare benign tumour. Usually these tumours are smaller than 5 cm (2); but, rare ones may become extremely large with extensive cystic changes (3, 4-6), as was noticed in this case. In such cases, massive enlargement occurs due to progressive degeneration and cyst formation with accumulation of fluid, rather than active growth of the tumour cells (4). The presence of hemorrhage and blood clots in a degenerated ovarian leiomyoma is another remarkable finding of our case, as few similar cases have described the presence of clear fluid in the cysts (3-5). This led us to presume the presence of endometriotic cyst, however, evidence of endometriosis was lacking on histology. Due to large size and the multiseptated mainly cystic nature, this lesion was initially misinterpreted as a malignant mass of the left ovary. Only after histopathologic examination a correct diagnosis was possible. The pseudomalignant appearance in this case may be attributed to unusual cystic degeneration of a leiomyoma. Interestingly, malignant-appearing complex cystic ovarian mass in the current case is proved to be a degenerated leiomyoma. We have confirmed the smooth-muscle origin of the tumour by desmin and SMA immunostaining which showed diffuse and strong cytoplasmic positivity, a characteristic immunoprofile of leiomyoma (7). Leiomyoma cell nuclei in this case have also shown significant positive staining for ER and PR on immunohistochemistry. The stimulatory effect of estrogen and progesterone on leiomyoma growth is well known. Progesterone can induce leiomyoma cell proliferation, suppress the stimulatory effects of estrogens in target tissues and downregulates ER-α expression, whereas estrogen upregulates the expression of PR (8). This may explain our observation of a stronger and more diffuse nuclear staining pattern of PR.
Conclusion
To summarize, we presented a case of clinically diagnosed malignant ovarian mass that proved to be a rare case of ovarian leiomyoma on histopathology. Extensive degeneration in an ovarian leiomyoma is unusual and one should consider this rare possibility whenever dealing with a cystic ovarian mass. We briefly discussed the pathogenesis with usefulness of histopathology and immunohistochemistry in establishing the correct diagnosis.
Acknowledgement:
The authors wish to thank Mr. Kanhaiya Lal and Mr. Vikash Agrawal for providing excellent technical assistance in immunohistochemical study of this case.
References:
[1].Robboy SJ, Mutter GL, Prat J, Bentley RC, Russell P, Anderson MC. Robboy’s pathology of the female reproductive tract. 2nd Ed. New York: Churchill Livingstone Elsevier; 2009
[2].Yoshitake T, Asayama Y, Yoshimitsu K, Irie H, Aibe H, Tajima T, Nishie A, Nakayama T, Kakihara D, Ariyoshi K, Kaneki E, Honda H. Bilateral ovarian leiomyomas: CT and MRI features. Abdom Imaging 2005;30:117-9..[pubmed][pubmed]
[3].Kim JC, Nam SL, Suh KS. Leiomyoma of the ovary mimicking mucinous cystadenoma. Clin Imaging 2000;24:34-7.[pubmed][pubmed]
[4].Wellmann KF. Leiomyoma of the ovary: report of an unusual case and review of the literature. Can Med Assoc J 1961;85:429-32.[pubmed][pubmed]
[5].Thamboo TP, Lee Y. Pseudocystic leiomyoma of the ovary: a case report and literature review. Pathology 2003; 35:534-5 [pubmed][pubmed]
[6].Kohno A, Yoshikawa W, Yunoki M, Yanagida T, Fukunaga S. MR findings in degenerated ovarian leiomyoma. Br J Radiol 1999;72:1213-5.[pubmed[ [pubmed]
[7].Lastarria D, Sachdev RK, Babury RA, Yu HM, Nuovo GJ. Immunohistochemical analysis for desmin in normal and neoplastic ovarian tissue. Arch Path Lab Med 1990;114:502-5[pubmed]
[8].Hermon TL, Moore AB, Yu L, Kissling GE, Castora FJ, Dixon D. Estrogen receptor alpha (ERα) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch 2008;453:557-69[pubmed][pubmed]

