Review

Cranioplasty : Routine Surgical Procedure or Risky Operation?
*Nicola Acciarri, *Francesca Nicolini, * Matteo Martinoni,
- *Department of Neurosurgery, IRCCS, Bellaria Hospital, Bologna, Italy
- Submitted Sunday, September 25, 2016
- Accepted Monday, October 24, 2016
- Published Wednesday, December 21, 2016
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Cranioplasty is a surgical procedure performed to restore a defect on the cranial vault after a previous decompressive craniectomy made for traumatic brain injury, ischemic or hemorrhagic disease, or even after the removal of cranial tumors. Although apparently it may resemble an easy and routine surgical procedure, cranioplasty has a rate of complications up to 41% of cases. The most frequently reported complications are infections, autologous bone flap resorption, and hematomas. Other possible complications are wound dehiscence, seizures, hygroma, and poor cosmetic results. In this paper we report an overview of the possible complications deriving from cranioplasty. The most evident causes of complications are discussed, suggesting, when possible, solutions to avoid or limit them.
Key Words:
Cranioplasty Complications, Infection, Bone Resorption, Haemorrhage, Seizures, Cosmetic Result
Introduction
Cranioplasty (CPL) it’s a secondary surgical procedure performed to restore a defect on the cranial vault after a previous operation made with the removal of skull bone flap. This commonly happens when a decompressive craniectomy is needed for brain edema due to traumatic injury, ischemic or haemorrhagic stroke, after the removal of cranio-dural tumors, or even after the correction of skull malformations. In many patients with severe neurological conditions, decompressive craniectomy is a life-saving procedure, but then it requires in survivors the bone flap replacement or its reconstruction with cranioplasty. Cranial reconstruction is important for several motives: it can provide protection to the underlying brain, may improve neurological function by recovering cerebro-spinal fluid (CSF) dynamics and cerebral blood flow, and it can restore cosmetically the cranial contour. Cranial reconstruction may be performed with autogenous and natural material, like the skull bone of the patient, or with alloplastic materials, like ceramics, acrylic resin, titanium, and others. Apparently, CPL may appear like an easy and routine surgical procedure, with few risks and possibly a low rate of complications.
Indeed, CPL is a risky surgical procedure, since at least one-third of cranial reconstructions are burdened by complications [1,2]. Several factors may influence the appearing of complications: time lapse between bone decompression and cranial reconstruction, materials used for CPL, age and conditions of patients, the experience of the surgeon on cranial reconstruction [3, 5].
Cranial reconstruction is a surgical procedure burdened by a high rate of complications, ranging between 15 and 41% of cases, in contrast with the lower complication rate of 2 - 5% for routine neurosurgical operations [6]. In addition, patients with CPL complications may need in 25-76% of cases of further surgical procedures to correct these complications, with a mortality rate over 3% of cases [7]. The causes for this high rate of CPL failure are not completely clear, since many factors may negatively influence the surgical results.
Complications after CPL are more frequently reported in male patients [6,11], and older age has been associated to a higher risk for CPL complications [11]. Some differences in the rate of complications have been described between procedures performed on the convexity, or in the suboccipital and bifrontal cranial locations [7].
The most frequent CPL complications reported in the literature are: infections, bone resorption, wound dehiscence, hemorrhage on or under the prostheses, seizures, hygromas [7 , 8,10, 12 ,16]. Although uncommonly mentioned [1, 11, 17], also poor cosmetic result must be recorded among possible CPL complications.
Infections
Infections due to CPL represent until more than 26% of cases [7 ,14] in contrast to a lower rate of 0.8% estimated for standard clean neurosurgical cranial procedures [3]. Infections are usually attributed to some relevant factors, like timing of surgery and the material used for CPL grafts. Commonly, after craniectomy, most of the cranial reconstructions are performed in a period ranging from 1 month to 1 year, without a specific consensus about the most proper timing for CPL. Replacement of cranial flap may be considered as “early” when performed before 3 months, “traditional” when performed between 3 and 6 months, and “delayed” when performed after 6 months [3]. Although recently it has been stated that patients submitted to CPL within 1-3 months after craniectomy are at higher risk (42% vs 13%) for infections respect to those operated on after 12 months [18], other recent studies [14, 15, 19, 20] have shown that an “early” surgery (within 3 months) does not entail differences in the risk rate of infections compared with “delayed” surgery. Furthermore, an early surgery may prevent also complications due to brain decompression [21], that in 2-29% may be complicated by CSF flow disorders [22], or other cerebral alterations, such as the “syndrome of the trephined” or the “sinking brain syndrome” [23 24].
Therefore, a CPL performed within a “traditional” time span, between 3 and 6 months after operculectomy craniectomy, is actually the most balanced line of action [25], since an “early” replacement of bone flap versus “delayed” implantation is not influencing the complication rate for infections [15], while an indolent infection in some cases may develop also after years from the cranial reconstruction.
Another supposed critical factor involved in the risk of infections is the material used for the cranial reconstruction. However, there is no strong evidence that allograft synthetic materials may predispose more than the autologous bone to infections [16,27]. On the contrary, the use of autologous bone in CPL does not guarantee a lower risk of complications, including infections that have been observed in 8-11% of cases [8, 19 28,30]. Probably, the general health state of patients is the most important factor, among those implied in the risk of infections after CPL. In fact, in patients with long hospitalization, immunocompromised by traumatic injuries and reoperations, the bacterial colonization of the skin is more easy [15]. This risk may be increased by simultaneous surgical procedures for hydrocephalus; therefore, concurrent surgical procedures for cranial reconstruction and CSF shunt are commonly discouraged [31]. The use of subcutaneous drain after CPL is controversial; surely, it’s not recommended in patients where the meningeal tissues presented a CSF leakage during the cranial reconstruction. Other factors reported in the literature as potentially causing an increasing risk of infections are: length of surgical time to perform the CPL; multiplicity of head lesions (skull fractures with meningeal and cerebral lacerations); traumatic or surgical violation of the frontal sinuses; width of cranial defect (bilateral craniectomy); older age; lack of pre- and postoperative antibiotic therapy [14, 15, 32]. Clinically, infections in patients submitted to a CPL usually present with spontaneous reopening of the surgical scar; in most typical cases, clinical signs of infection are represented by scalp tenderness with pain, redness, and swelling over the implant (Figure 1). Diagnosis of infection may be accomplished with specific wound specimens for determination of the bacterium, and from the careful analysis of laboratory inflammatory markers on patient blood samples: white blood cell count, erythrocyte sedimentation rate, and C-reactive protein [3]. Once the infection is detected, these markers may be used also to determine if an antimicrobial therapy is the most appropriate [33].
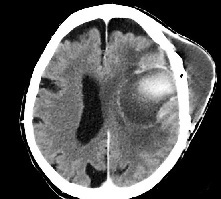
Figure 1: Computed Tomography ( CT ) scan image of a left frontal cerebral abscess in patient with previous CPL
Prevention of infections, however, must be done at time of surgical brain decompression: in case of wide craniectomy for traumas, a large spectrum antibiotic therapy for patients is mandatory in the pre- and postoperative course until tissue specimen or blood bacterial examinations lead to a specific antimicrobial therapy. In patients who had gross contamination of the scalp at the time of the head trauma and subsequent craniectomy, it may be best to delay the CPL until head skin conditions and the neurological recovery of the patient are adequate to surgical timing
Bone resorption
In case of cranial reconstruction, autologous bone is commonly considered the most biocompatible material, and its use has been advocated instead of allograft prosthesis [34]. Once bone decompression is performed, the autologous flap may be stored in the subcutaneous fat, usually in the abdominal wall, or in a freezer, after adequate sterile wrapping, until the time of replantation. Compared to other materials, autologous bone is economically advantageous, although the cost of cryopreservation. Unfortunately, complications due to autologous bone represent on average 29-31% of cases of CPL [19], with a general resorption rate of 21% [22, 27]. Younger age, bone flap fragmentation, and shunt-dependent hydrocephalus have been reported as adjunctive risk factors for bone resorption [35] that is radiologically and clinically evident in 3-12 months from the cranial reconstruction (Figure 2).
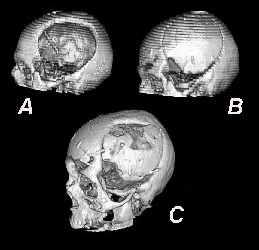
Figure 2: Bone flap resorption sequence on CT scan in a patient submitted to cranial reconstruction with autologous bone flap. A) - CT scan showing a left fronto-parietal cranial defect after brain decompression for head trauma. An external CSF drainage is visible inside the skull. B) - Same patient, six months later, after cranial reconstruction with cryopreserved autologous bone flap. C) – Ten months later a CT scan is clearly showing the bone flap resorption, causing further reoperation in the patient.
Among the possible causes of complications with the use of autologous bone, its storage methods after craniectomy have to be considered: preservation methods, in the subcutaneous fat and the cryopreservation, are both burdened by problems, and bone resorption is the most common critical one. While the risk rate for autologous bone resorption in adults has been estimated in 3-12% of cases [12], this rate may rise up to 50% of cases for pediatric patients [22, 36 37]. Although the bone capacity to regenerate itself is higher in the pediatric age, this may be hindered by the volumetric development of intracranial structures that occurs for the 80% during the first two years of life. Therefore, the bone healing process between two fragments that tend to move away from each other may be limited by the brain development [38]. As a consequence, the use of acrylic resin has been suggested not only in adults, but also in pediatric patients for its good results and the low rate of complications [39,40], although ceramics and hydroxyapatite materials may be considered the most suitable substitutes of autologous bone. The mechanisms involved in the autologous bone resorption are probably based on multiple factors [41]: the traumatic or non-traumatic origin of the bone flap, the storage method, the length of the storage, the size of it.
It has been observed that bone flaps coming from a cranial decompression due to trauma are more prone (8.5% vs 1.8%) to resorption than those coming from decompression for other motives [37]; similarly, bone resorption seems higher when flap is stored in the abdominal wall [42]. Indeed, many traumatic bone flaps are originally damaged and their recover based on the osteoconduction is impossible when they are detached from surrounding resident skull bone. However, there is no proved evidence that opercula coming from non-traumatic decompressions are less prone to resorption. Probably, when bone is storaged some biological deterioration may happen in time, and this is in accord with the observation that bone flaps cryopreserved for more than 6 weeks tend to resorb more easily [43].
Also the size of the flap may negatively influence bone replantation: it seems that flaps larger more than 70 cm² tend more easily to resorb [44]. Furthermore, when the autologous bone is cryopreserved doubts persist as to whether the degree of sterilization is adequate, and there is no good way to sterilize an autologous bone without predisposing the flap to resorption. The temperature of cryopreservation is usually between -35 and -80 C°, which may preserve the bone from infections, but probably is causing a biological tissue alteration when the bone is defrosted. Finally, also the removal from its anatomical site and its handling during surgery may negatively affect the original bone structure, causing the basis for the flap resorption [35]; once the bone flap is replanted, beside the risks of biological alterations from storage, resorption may be promoted also by its temporary mobilization. Since the infection risk rate of autologous bone seems to be similar to that of allograft material [8, 29, 30], the postoperative risk of hematoma is not inferior to that of synthetic material [31], the long-term failure of autologous bone is possible [19], and the outcome of patients treated with synthetic material seems to be better than that of patients treated with autologous bone [45], it’s natural to wonder if it is worth the use of allograft synthetic material from the beginning instead of the autologous bone in case of a cranial reconstruction. Some suggestions may derive from these considerations: the bone flap must be carefully fixed to the edges of the cranial defect in order to avoid pseudoarthrosis, and facilitating osseous fusion; in case of a very large decompression defect, a custom-made prosthesis is recommended
Wound Dehiscence
Wound dehiscence caused by a prosthesis represents more than 4% of CPL complications [46] and is mostly due to the use of prosthetic materials that are corrosive for the skin (steel mesh, protruding metal plates, etc.), or due to implant dislodgement [9]. A debilitated physical condition, previous skin irradiation or inadequate care of the surgical wound over the prosthesis may facilitate the formation of dehiscence from a decubitus skin area. This should be avoided since a skin dehiscence may evolve into infection in 0.7% of cases [11].
Infections are often attributable to saprophytes that can more easily exert their pathogenic action when the patient is debilitated by a long-term hospitalization. Therefore, many cases of dehiscence from CPL procedures may be avoided by paying attention to limit the metallic material used during the cranial reconstruction and, overall, to the skull points where this material is positioned, usually to fix the prosthesis. Furthermore, good care of surgical wound with cleaning of the skin flap may dramatically reduce the risk of infections that develop from wound dehiscence.
Hematoma
Hemorrhage over or under a prosthesis is diagnosed in 1.8 - 12.24% of all CPL procedures [1, 2, 8, 11]. However, not all the postoperative hematomas require a new surgical revision, because they are often tolerated (Figure 3). In fact, many patients submitted to cranial reconstruction have an atrophic brain from a previous traumatic lesion or stroke; as a consequence, the residual cavity underneath the CPL may be easily filled up with postoperative hematoma. Usually, the risk of hemorrhage is not correlated to the material used for the prosthesis, although some authors reported a higher rate (20% of cases) of hematomas in a series with autologous bone [8]. Extradural hemorrhage may occur in the early postoperative period, representing 1.65 - 3.13% of surgical CPL complications [1, 14, 47]. Commonly, risk factors for hemorrhage after CPL are: surgical manipulation of soft tissues (muscles, subcutaneous layers) without a careful hemostasis; dural compression by prosthesis with risk of cerebral lesions; blood loss from bone edges of the skull defect; anticoagulant-antiplatelet therapy not preventively discontinued before the cranial reconstruction, or shortly resumed after. The most important factors remain the persistent bleeding by scalp arteries along with a negative transluminal pressure caused by a subgaleal drain [7 ,47], although it’s controversial whether an extradural hematoma may be adequately prevented by the use of subcutaneous drainage [1, 10].
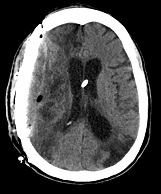
Figure 3: Postoperative CT scan in a patient submitted to right fronto-parietal CPL. Extradural blood collection is visible under the prosthesis, although patient resulted asymptomatic from this hematoma.
Seizures
Seizures after cranial reconstruction may range from 0.45% [2] to almost 15% of cases [1, 7 14,16]; however, in some series seizures are not reported among CPL complications, but are attributed to pre-existing causes [10]. Bifrontal cranial reconstruction and old age have been reported as factors predisposing to seizures [7]. Clinically, seizures can be considered “immediate” when appearing within 24 hours after surgery (12.5% of cases), “early”, the most rare, when appearing within 7 days from surgery ( 3.12% of cases), or “delayed”, the most frequent, when appearing after 7 days (over 40% of cases) [14]. Seizures are thought to be triggered by the surgical manipulation of the brain during cranial reconstruction, increasing its epileptogenic susceptibility and probably altering other factors in CSF dynamics [16]. In case of traumatic cranial defects, seizures have mostly an early onset and may be caused by ischemic or edematous cerebral alterations, together with axonal injury and cerebral distortion [14]. In patients that underwent craniectomy for non- traumatic reasons (tumor, hemorrhage, stroke) seizures are mostly delayed, and may be caused by gliosis, residual hemosiderin, or other inflammatory factors [14]. Prevention of seizures with antiepileptic therapy before cranial reconstruction is still controversial; in the past, antiepileptic therapy was delivered when the postoperative epilepsy rate exceeded 10-15% of cases [48]. Actually, the standard line of therapy has changed: perioperative prophylactic antiepileptic therapy should be carefully considered only in patients with documented history of epilepsy. In order to decrease the risk of seizures after cranial reconstruction, it’s recommended to avoid or reduce as much as possible the manipulation of the dura mater when preparing the skull edges of the cranial defect, paying attention also to not compress the brain by forcing the placement of the prosthesis.
Hygroma
Hygromas following a cranial reconstruction represent around 2.2% of CPL complications and are usually associated with CSF abnormalities due to a CSF shunt or a pre-existing subdural hematoma [11]. Commonly, hygromas may be controlled by radiological follow-up and rarely have to be treated with a new operation; however, when a hygroma appearing after CPL is related to a pre-existing CSF shunt, dramatic intracranial hemorrhage may be avoided up-regulating the shunt valve pressure. Furthermore, concurrent operations for CSF shunting and cranial reconstruction have a higher risk of infections [31]; therefore, these operations should be performed in two different surgical steps. The first one should be the CPL, and the second one, if still needed after a while, the correction of the hydrocephalus. In fact, the cranial reconstruction is able in many cases to restore CSF dynamics, improving also cerebral blood flow and neurological brain functions.
CSF collection
This is a common complication of cranial reconstruction in patients with traumatic damage of meningeal tissues or in patients who underwent several surgical procedures, in whom a dural tearing with CSF leakage may present with pseudomeningocele [1]. However, the surgical revision is not always needed, since the installation of a CSF spinal drainage in the patient, eventually aided from head bandage, may often resolve this complication. The presence of subcutaneous CSF may increase infection risks; therefore, any surgical manoeuvre that may damage dura mater during the CPL should be avoided, especially when preparing the skull edges of the cranial defect. Finally, when performing craniectomy, the use of further synthetic dural layer over the original one proves to be helpful, allowing an easier detachment of the skin flap from the cranial defect during cranial reconstruction [1, 49].
Poor cosmetic result
After a cranial reconstruction, poor cosmetic result is possible; however, in the literature this complication is rarely mentioned, probably because in many cases hair and soft tissues of head are covering the unsightly area. In the literature, poor cosmetic results have been recorded from 1.5 to 8.7% of cases [1,11,17], and may be caused by several factors: the irregular modelling of acrylic resin may result in an inadequate prosthesis; in case of autologous bone, the resorption may cause the sinking of the flap; in every case of malpositioned prosthesis a poor cosmetic result is possible. However, poor cosmetic results are probably underscored and rarely reported in the literature, because there is the general conviction that the aesthetic result after CPL, especially in patients with bad neurological state, is less important than improvement of neurological brain functions and quality life. Furthermore, it has not been proposed yet in the literature a scientifically accepted method of evaluation of cosmetic results that may be suitable for all surgical centers performing CPLs, and even harder is to propose a method based on a computerized comparison between the real cranial reconstruction and the one ideally designed. On the other hand, when taken into account the cosmetic results, these should be based also on degree of satisfaction expressed by patients, but this judgment is not always available in an adequate manner due to the neurological inability of some patients or their refusal to provide an opinion. It’s our opinion that in patients with good neurological conditions, especially young people with a socially active life, the aesthetic result remains one of the most important goals to be achieved with cranial reconstruction. In case of CPL with acrylic material, some technical tips for a successful procedure may be adopted [50], but the surgeon’s skill remains one of the most critical factors for the achievement of cosmetically adequate results. We suggest also the maximum care in positioning the prosthesis in the cranial defect, since implant dislodgement is possible in more than 3% of cranial reconstructions [9].
Undoubtedly, for a better cosmetic result the cranial reconstruction should be made with custom-made prosthesis.
Conclusions
From this review of the literature, it seems well established that CPL is a surgical procedure affected by high rate of complications. Risks for complications may derive from surgery timing, inadequate prosthetic material, concurrent CSF abnormalities or systemic diseases.
Nevertheless, CPL is too often considered an easy and routine surgical procedure, occasionally performed by surgeons not very skilled with cranial reconstruction, and without the supervision of an experienced surgeon. This may cause a suboptimal surgical management, increasing the risk for complications and possibly conditioning the cosmetic result, especially with materials such as acrylic resin or autologous bone. Indeed, cranial reconstruction is a delicate type of operation that should be carried out with meticulous surgical technique, not underestimating in patients their general health state, neurological conditions, and head skin integrity around the cranial defect. Only with the careful evaluation of risk factors for failure, and substantial surgical experience, CPL morbidity and complications may be reduced.
Abbreviations
Cranioplasty –CPL; Cerebro-spinal fluid- CSF; Computed Tomography-CT
Learning Points
Cranioplasty is too often considered a routine surgical procedure, with low risks, while indeed is a challenging operation, with high rate of complications
Complications from Cranioplasty are believed more frequent with alloplastic materials, while indeed a high rate of complications is possible also with autologous bone
Since Cranioplasty is considered an easy surgical procedure, it is performed often by surgeons not skilled with cranial reconstruction, and this may rise the rate of complications, including also poor cosmetic results
Conflict of Interest
The authors declare that there is no conflict of interest
Authors’ Contributions
NA: data collection and management; manuscript writing and editing.
FN: data collection and management.
MM: data collection and management.
Funding Source
None declared
References
[1]Coulter IC, Pesic-Smith JD, Cato-Addison WB, Khan SA, Thompson DT, Jenkins AJ, et al. Routine but risky: A multi-centre analysis of outcomes of cranioplasty in the Northeast of England. Acta Neurochir 2014; 156:1361-8.[Pubmed]
[2].Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus 2009; 26: E9 [Pubmed]
[3]Hall WA. Cranioplasty Infections – Adding Insult to Injury. World Neurosurgery 2014; 82: E 435-7[Pubmed]
[4]Joswig H, Gautschi OP, El Rahal A, Sveikata L, Bartoli A, and Hildebrandt G, et Al. Cranioplasty: Is surgical education safe? World Neurosurg 2016; 91:81-8 [Pubmed]
[5]Liang FS, Tipper G, Hunt L, Gan PYC. Cranioplasty outcomes and associated complications: a single-center observational study. Br J Neurosurg 2016; 30:122-7 [Pubmed]
[6].Godil SS, Shamim MS, Enam SA, Qidwai
U, Qadeer M, Sobani ZA. Cranial reconstruction after decompressive craniectomy:
prediction of complications using fuzzy logic. J Craniofac Surg. 2011
Jul;22(4):1307-11. doi: 10.1097/SCS.0b013e31821c6d37. [Pubmed]
[7]Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M, et al. Complications following cranioplasty: incidence and predictors in 348 cases. J Neurosurg 2015; 123:182-8. [Pubmed]
[8].Bobinski L, Koskinen L-OD, Lindvall P. Complications following cranioplasty using autologous bone or polymethymethacrylate – Retrospective experience from a single center. Clin Neurol and Neurosurg 2013; 115:1788-91 [Pubmed]
[9]Brommeland T, Rydning PN, Pripp AH, Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med. 2015; 23: 75[Pubmed]
[10].Klinger DR, Madden C, Beshay J, White J, Gambrel K, Rickert K. Autologous and Acrylic Cranioplasty: a review of 10 years and 258 cases. World Neurosurgery 2014; 82:E525-30 [Pubmed]
[11].Wachter D, Reineke K, Behm T, Rohde V. Cranioplasty after decompressive hemicraniectomy: Understimatedd surgery-associated complications? Clin Neurol and Neurosurg 2013; 115:1293-97.
[Pubmed]
[12].Honeybull S, Ho KM. Long term complications of decompressive craniectomy for head injury. J of Neurotrauma 2011; 28:929-35 [Pubmed]
[13].Honeybull S, Morrison DA, Ho K, Wiggins A, Janzen C, Kruger K. Complications and consent following decompressive craniectomy: An illustrative case study. Brain Injury 2013; 27:1732-36 [Pubmed]
[14]Lee L, Ker J, Quah BL, Chou N, Choy D, Yeo TT. A retrospective analysis and review of an institution’s experience with the complications of cranioplasty. Br J of Neurosurg 2013; 27: 629-35 [Pubmed]
[15]Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of Flap preservation on Cranioplasty Infections: A systemic review. Neurosurgery 2011; 68:1124-30.[Pubmed]
[16].Walcott BP, Kwon C-S, Sheth S, Fehnel CR, Koffie RM, Asaad WF, et al. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg 2013; 118:757-62 [Pubmed]
[17].Fischer CM, Burkhardt JK, Sarnthein
J, Bernays RL, Bozinov O. Aesthetic outcome in patients after
polymethyl-methacrylate (PMMA) cranioplasty - a questionnaire-based
single-centre study. Neurol Res. 2012 Apr;34(3):281-5. doi:
10.1179/1743132812Y.0000000007. Epub 2012 Mar 9. [Pubmed]
[18].Borger V, Schuss P, Kinfe , Vatter H, Guresir E. Decompressive craniectomy for stroke: Early Cranioplasty is a predictor for complications? World Neurosurg 2016; 92:83-8 [Pubmed]
[19].Honeybull S, Ho KM. How “successful” is calvarial reconstruction using frozen autologous bone? Plast Reconstr Surg 2012; 130:1110-17.[Pubmed]
[20].Quah BL, Low HL, Wilson MH, Bimpis A, Nga VDW, Lwin S, et Al. Is there an optimal time for performing cranioplasties? Results from a Prospective multinational study. World Neurosurg 2016; 94:13-7 [Pubmed]
[21].Songara A, Gupta R, Jain N, Rege S, Masand R. Early Cranioplasty in patients with posttraumatic decompressive craniectomy and its correlation with changes in cerebral perfusion parameters and neurocognitive outcome. World Neurosurg 2016; 94:303-8.
[Pubmed]
[22].Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus 2009; 26: E7.[Pubmed]
[23].Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg 1939; 110:488-512.[Pubmed]
[24].Yamamura A, Sato M, Meguro K, Nakamura T, Uemura K, Makino H. Cranioplasty following decompressive craniectomy. Analysis of 300 cases. No Shinkei Geka 1977; 5:345-53 [Pubmed]
[25].Stiver SI, Wintermark M, Manley GT. Reversible monoparesis following decompressive hemicraniectomy for traumatic brain injury. J Neurosurg 2008; 109:245-54.
[Pubmed]
[26].Huang APH,Tsai JG, Kuo LT, Lee CW, Lai HS, Tsai L-K, et al. Clinical application of perfusion computed tomography in neurosurgery. J Neurosurg 2014; 120:473-88 [Pubmed]
[27].Malliti M, Page P, Gury C, Chomette E, Nataf F, Roux FX. Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery 2004; 54:599–604.
[Pubmed]
[28].Chibbaro S, Di Rocco F, Mirone G, Fricia M, Makiese O, Di Emidio P, et al. Decompressive craniectomy and early cranioplasty for the management of severe head injury: a prospective multicentre study on 147 patients. World Neurosurg 2011;75: 558-62 [Pubmed]
[29]Sundseth J, Sundseth A, Berg-Johnsen J, Sorteberg W, Lindegaard KF. Cranioplasty with autologous cryopreserved bone after decompressive craniectomy. Complications and risk factors for developing surgical site infection. Acta Neurochir 2014;156:805–11.
[Pubmed]
[30].Werndle MC, Crocker M, Zoumprouli A, Papadopoulos MC. Modified acrylic cranioplasty for large cranial defects. Clin Neurol Neurosurg 2012; 114:962-4
[Pubmed
[31].Schuss P, Borger V, Güresir Á, Vatter H, Güresir E. Cranioplasty and ventriculoperitoneal shunt placement after decompressive craniectomy: staged surgery is associated with fewer postoperative complications. World Neurosurg 2015; 84:1051- 4.
[Pubmed]
[32].Im SH, Jang DK, Han YM, Kim JT, Chung DS, Park YS. Long-term incidence and predicting factors of cranioplasty infection after decompressive craniectomy. J Korean Neurosurg Soc 2012;52:396–403. [Pubmed]
[33].Le C, Guppy KH, Axelrod YV, Hawk MW, Silverthorn J, Inacio MC, et al. Lower complication rates for cranioplasty with peri-operative bundle. Clin Neurol Neurosurg 2014; 120:41-4 [Pubmed]
[34Lemée JM, Petit D, Splingard M, Menei P. Autologous bone flap versus hydroxyapatite prosthesis in first intention in secondary cranioplasty after decompressive craniectomy: a French medico-economical study. Neurochirurgie 2013; 59:60-3.
[Pubmed]
[35].Schwarz F, Dünisch P, Walter J, Sakr Y, Kalff R, Ewald C. Cranioplasty after decompressive craniectomy: is there a rationale for an initial artificial bone-substitute implant? A single-center experience after 631 procedures. J Neurosurg 2016;124:710-5.
[Pubmed]
[36].Martin KD, Franz B, Kirsch M, Polanski W, Von der Hagen M, Schackert G, et al. Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir 2014; 156: 813-24.
[Pubmed]
[37].Schuss P, Vatter H, Oszvald A, Marquardt G, Imöhl L, Seifert V, et al. Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma 2013; 30:91-5.
[Pubmed]
[38].Bowers CA, Riva-Cambrin J, Hertzler DA, Walker ML. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr 2013;11: 526-32.
[Pubmed]
[39].Fiaschi P, Pavanello M, Imperato A, Dallolio V, Accogli A, Capra V, et al. Surgical results of cranioplasty with a polymethylmethacrylate customized cranial implant in pediatric patients: a single-center experience. J Neurosurg Pediatr 2016;17:705-10.
[Pubmed]
[40]. Fu JK, Barr RM, Kerr ML, Shah MN, Fletcher SA, Sandberg DI, et Al. An outcomes comparison between autologous and alloplastic cranioplasty in the pediatric population. The Journal of craniofacial surgery 2016; in press : DOI: 10.1097/SCS.0000000000002491
[Pubmed]
[41].Kim JS, Cheong JH, Ryu JI, Kim JM, Kim CH. Bone Flap Resorption Following Cranioplasty after Decompressive Craniectomy: Preliminary Report. Korean J Neurotrauma 2015;11:1-5.
[Pubmed]
[42].Inamasu J, Kuramae T, Nakatsukasa M. Does Difference in the Storage Method of Bone Flaps After Decompressive Craniectomy Affect the Incidence of Surgical Site Infection After Cranioplasty? Comparison Between Subcutaneous Pocket and Cryopreservation. J of Trauma-Injury Infection & Critical Care 2010; 68:183-7.
[Pubmed]
[43].Piedra MP, Thompson EM, Selden NR, Ragel BT, Guillaume DJ. Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr 2012; 10:268-72.
[Pubmed]
[44].Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg 2004;100:163-8 [Pubmed]
[45].Piitulainen JM, Kauko T, Aitasalo KM, Vuorinen V, Vallittu PK, Posti JP. Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg 2015;83:708-14.[Pubmed]
[46].Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg 2003; 14:144-53.[Pubmed]
[47].Roth J, Galeano E, Milla S, Hartmannsgruber MW, Weiner, Howard L. Multiple Epidural Hematomas and Hemodynamic Collapse Caused by a Subgaleal Drain and Suction-Induced Intracranial Hypotension: Case Report. Neurosurgery 2011; 68:271-6.
[Pubmed]
[48].Deutschman CS, Haines SJ. Anticonvulsant Prophylaxis in Neurological Surgery. Neurosurgery 1985;17:510-7.[Pubmed]
[49].Oladunjoye AO, Schrot RJ, Zwienenberg-Lee M, Muizelaar JP, Shahlaie K. Decompressive craniectomy using gelatin film and future bone flap replacement. J Neurosurg 2013;118:776-782.[Pubmed]
[50].Han SE, Lim SY, Pyon JK, Mun GH, Bang SI, Oh KS. Aesthetic refinement of secondary cranioplasty using methyl methacrylate bone cements. Aesthetic Plast Surg. 2013;37:592- 600.
[Pubmed]

