Case Report

Staged hemispherotomy in an infant with catastrophic epilepsy without hemiparesis
1,Alaa Eldin Elsharkawy, 2Ingrid Tuxhorn
- 1University Clinic, arnold-heller-str. 3, 24105 Kiel, Germany
- 2Cleveland Clinic, Epilepsy Center, USA
- Submitted Sunday, July 06, 2014
- Accepted:Friday, July 25, 2014
- PublishedSunday, August 03, 2014
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Objective
Timing of hemispherotomy in young children with catastrophic epilepsy is a clinical challenge for the surgical and pediatric team.
Clinical Presentation
a 4-year-old boy presented at age of 3.5 weeks with catastrophic right temporo-parieto-occipital epilepsy, without motor neurological deficit. Magnetic resonance imaging (MRI) showed right temporo-parieto-occipital focal cortical dysplasia (FCD). Video EEG monitoring was concordant for seizure onset with the MRI lesion.
Intervention
The patient underwent temporo-parieto-occipital disconnection at 7 months of age, extended disconnection at 11 months and a multilobar resection at 14 months of age there was only temporary seizure reduction. Repeat presurgical evaluation at 20 months of age demonstrated more extensive dysplasia involving the right frontocentral regions. At age of 22 months the patient became seizure free after right hemispherotomy. Postoperatively he became alert and had restart of development but motor examination showed a moderate left hemiparesis.
Conclusion
this case demonstrates that an aggressive approach in infants with epileptic encephalopathy due to extensive FCD is warranted. Assessment of the risk/benefit ratio for hemispherotomy should be individualized and each patient must be assessed separately
Introduction
Hemispherectomy (anatomical and functional) may control seizures in 54 to 88% of children suffering from catastrophic epilepsy [1, 2, 3, 4, 5, 6]. Decision to perform hemispherectomy in catastrophic epilepsy is based on three outlined selection criterions: pharmacoresistancy of the epilepsy syndrome, hemiparesis contralateral to the epilepsy causing hemisphere, and structural and functional changes must be found in the contralateral hemisphere. In addition, a low risk of new unacceptable postoperative neurologic deficits [2, 7, 9].
However patient selection and the optimal timing of surgery remain a challenge in the very young and ill infant due to the increased operative risks of the procedure related to blood loss and technical complexities affecting outcome and related to the etiology and pathology of extensive malformative lesions [10-12]. The presurgical evaluation with video EEG and imaging frequently may not define the extent of the epileptigenic lesion and immature myelination patterns may lead to poor demarcation of the dyspasia on MRI with underestimation of brain involvement [13].
Case Presentation
A 4-year-old boy presented in our clinic at age of 3.5 weeks with catastrophic epilepsy. Pregnancy, labor and delivery were normal. Family history was positive for a cousin of the mother who had epilepsy since age of 30 years. Neurological examination showed no neurological deficit.
Initial seizure assessment showed an infantile spasms and focal motor seizures with left sided clonic-myoclonic jerking with seizures frequency in the range of 30-50/day. At least four different antiepileptic drugs were tried without any improving. The Patient has been referred to presurgical evaluation where the standard Video-EEG Monitoring, MRI and neuropsychological evaluation were performed.
The Video-EEG Monitoring showed in the interictal and ictal EEG a right temporo-occipital EEG seizure patterns (Figure 1) MRI showed a FCD right temporo-occipital and parieto-mesial as well as temporo and occipito-lateral (Figure 2) Temporo-parieto-occipital epilepsy has been diagnosed and confirmed by concordance between MRI, interictal and ictal EEG.
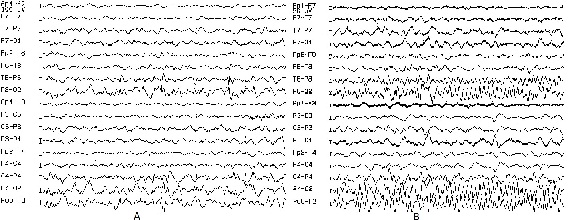
Figure 1: Video-EEG Monitoring showing the interictal and ictal EEG a right
temporo-occipital EEG seizure patterns
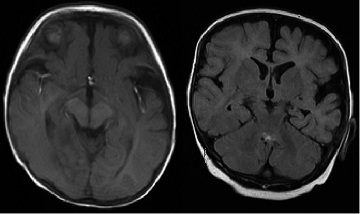
Figure 2: MRI showing a FCD right temporo-occipital and parieto-mesial as
well as temporo and occipito-lateral
First and second surgical Intervention
The case has been presented in the multidisciplinary case conference for discussion.The decision was to perform a temporo-occipital disconnection based on the absence of motor defects and MRI with FCD. At age of 8 months, the temporo-occipital disconnection according to the technique described by Villemure was performed [14 15]. The histo-pathology showed a type IIa dyspasia based on the classification of Palmini et al. postoperatively the patient had a transient seizure reduction for only a few weeks.
The case has been presented again in the multidisciplinary case conference for discussion. Extension of the temporo-occipital disconnection has been indicated. Four months later the extension of the temporo-occipital disconnection was performed. Postoperatively again the patient had a transient seizure reduction for 3 weeks. Postoperatively the patient developed transient right-side hemiparesis and transient supraventricular tachycardia of undetermined etiology despite extensive evaluation. MRI showed left MCA ischemic infarction, paresis was transient and the patient recovered completely within weeks after.
Third surgical intervention
A second video EEG monitoring showed again a right tempro-occipital seizure pattern with hypomotor, tonic and clonic facial seizures in clusters lasting 1.5 hours. MRI showed a FCD in temporo-parieto-occipital region with chronic subdural hematom.
A multilobar resection of the temporo-parieto-occipital region up to the motor strip was performed at 15 months of age. A subdural hematoma was evacuated perioperatively. Postoperatively, no improvement of the seizures was noted, but the seizure semiology showed several changes. . The patient appeared to have a somatosensory aura in the left hand preceded seizures as he wold take hold of the hand. Hypomotor seizures (40/day), tonic seizure and myoclonic attack of the left arm have been recorded.
Hemispherotomy
Third time Video EEG Monitoring showed in the interictal EEG: Polyspikes right frontal, spikes left frontal, and sharp waves left frontal. Ictal EEG showed right frontal –> left temporal EEG-seizure pattern. MRI showed residual right parieto-frontal-parasagittal FCD extending anterior to the resection line into the central region.
Based on these findings the family was offered a hemispherotomy to disconnect the remaning frontocentral region with the understanding that he was very likely to loose motor function of the left side.
A right-side Hemispherotomy was performed at age of 22 months. Our technique is a modified technique of Delalande [16],
it is a combined lateral and vertical hemispherectomy technique(Figure. 3). [2, 6] . Postoperatively, the patient became immediately seizure free, had a left side hemiparesis and required implantation of ventriculo-peritoneal shunt for post operative hydrocephalus.
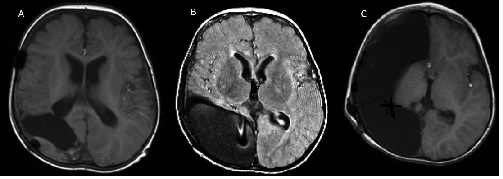
Figure 3: MRI showing right-side Hemispherotomy
In follow up over 2 years the patient is seizure free since hemispherotomy under Oxcarbazepin monotherapy. He is able to walk, eats independently with cutlery, and shows better speech understanding and speaking single words well understood, he started his first 2-word phrases. He is interested in colors and begins to figurative drawing.
Neuropsychological outcome
The Bayley Scales of Infant Development second edition (BSID-II), mental scale record was used to evaluate the patient’s mental development before and after surgery, at age of 1.1 year (after disconnection operation) the patient was at the 4 month developmental level, his mental development stopped at this level up to age of 22 months. Before hemispherotomy, due to worsening of the seizures condition, the patient was unable to be examined. Two years after hemispherectomy, his BSID-II score was equal to 23 months.
Discussion
Early surgery has been advocated for children with refractory focal epilepsy to improve seizure outcome and potentially reduce the risk of longterm developmental morbidity [17]. Previous studies therefore advocate that children should be considered for surgical evaluation at an early age if they manifest with severe, intractable, disabling localization-related epilepsy [11 18]. Seizures among children with uncontrolled epilepsy significantly increase the risk of seizure-induced brain damage and regression in mental and cognitive development [9 19 35]. Delaying the timing of surgery may reduce the recovery potential of mental and cognitive function as a recent study demonstrated a significant correlation of gain in DQ point at 2 years after surgery in relation to duration of the epilepsy [11,17].
In the assessment of the risk/benefit ratio for surgery 3 basic questions should be outlined; the chance for seizure freedom; risks of surgery and risk of not doing surgery. Developmental prognosis and age–related issue is a cornerstone in the analysis of the potential risks and benefits of epilepsy surgery [9]. In our case, the difficult question was, should hemispherotomy be performed? Or should we wait until hemiplegia is complete? Presence of hemiplegia and hemianopias make the decision to perform hemispherotomy is much easier [9]. Despite absence of hemiplegia our multidisciplinary team found that hemispherectomy is the last option for this patient and hemiplegia will occur anyway later in the course of the disease.
Reviewing of literature was supporting to this decision, it has been reported that onset of seizures before 2 years of age, as in our case, is a risk factor for subsequent mental retardation independent of etiology [20] 21. Mental and cognitive regression were more prominent in patients with dysplasia [22 23]. Presence of FCD explain the failure of the first three surgical intervention in our case report, it has been reported the FCD can be identified accurately after 24 months of age [13], despite this fact, it has been documented that early surgery in those group of patient can allow the resumption of more normal development [23]. In general, best developmental outcome and cognitive outcome were noted in patients with earliest surgery and shorter duration of seizures until surgery [19 24 27]. A theoretical benefit exists to performing early surgery to promote better behavioral and cognitive development, taking advantage of neuroplasticity that is still present in early life [28] and preventing detrimental effects of severe and frequent seizures on neuro developmental progress [29].
Reported outcome after hemispherectomy in patients with catastrophic epilepsy provides excellent seizure outcome with a satisfactory complication rate[2 7 8 29 30]. Developmental progress after hemispherectomy in patient with catastrophic epilepsy has been documented [22 23.30 33]
hemispherectomy has been reported in non-classical indications, if the patient has so severe as to be life-threatening seizures, there is markedly progressive developmental delay, and all other therapeutic options were failed to control the seizure [23 34]. Even when an occasional ictal event is seen to originate from the contralateral hemisphere [34].
Conclusions
Assessment of the risk/benefit ratio for hemispherectomy should be individualized and each patient must be assessed separately. In children with catastrophic epilepsy who fail a first surgery, early thought should be given to re-evaluation and re-operation. In addition a more aggressive approach may be warranted with early hemispherectomy in multilobar cases to improve overall prognosis.
Authors’ Contributions
AEE: carried out the design of the work,
analysis and interpretation of data for the work, drafting the work, literature
search and final approval of the version to be published.
IT: carried out the English correction of
the draft, EEG interpretation and Final approval of the version to be published.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Ethical Considerations
Written informed consent was obtained from the patient for
publication of this case report.
Acknowledgement
None
Funding
None declared
References
[1]Lassonde M, Sauerwein HC, Jambaque I, Smith ML, Helmstaedter C. Neuropsychology of childhood epilepsy: pre- and postsurgical assessment. Epileptic Disord l2000;2: 3-13.[Pubmed]
[2]Elsharkawy AE, Pannek H, Oppel F. Hemispherectomy. Pan Arab Journal of Neurosurgery l2008;12: 11-18.
[3]Kanev PM, Foley CM, Miles D. Ultrasound-tailored functional hemispherectomy for surgical control of seizures in children. J Neurosurg l1997;86: 762-7[Pubmed]
[4]Schramm J. Hemispherectomy techniques. Neurosurg Clin N Am l2002;13: 113-34, ix[Pubmed]
[5]Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery l2001;49: 891-900; discussion 900-1.[Pubmed]
[6]Schramm J, Behrens E. Functional hemispherectomy. J Neurosurg l1997;87: 801-2.[Pubmed]
[7].]Peacock WJ, Wehby-Grant MC, Shields WD, Shewmon DA, Chugani HT, Sankar R, Vinters HV. Hemispherectomy for intractable seizures in children: a report of 58 cases. Childs Nerv Syst l1996;12: 376-84[Pubmed]
[8] Wyllie E. Surgical treatment of epilepsy in children. Pediatr Neurol l1998;19: 179-88.[Pubmed]
[9]Wyllie E. Catastrophic epilepsy in infants and children: identification of surgical candidates. Epileptic Disord l1999;1: 261-4.[Pubmed]
[10]Wyllie E. Surgery for catastrophic localization-related epilepsy in infants. Epilepsia l1996;37 Suppl 1: S22-5.[Pubmed]
[11].Oguni H, Mukahira K, Tanaka T, Awaya Y, Saito K, Shimizu H, Oda M, Arai N, Suzuki I, Osawa M. Surgical indication for refractory childhood epilepsy. Epilepsia l2000;41 Suppl 9: 21-5[Pubmed]
[12]Devlin AM, Cross JH, Harkness W, Chong WK, Harding B, Vargha-Khadem F, Neville BG. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain l2003;126: 556-66[Pubmed]
[13]Woermann FG, Vollmar C. Clinical MRI in children and adults with focal epilepsy: a critical review. Epilepsy Behav l2009;15: 40-9.
[14]Daniel RT, Meagher-Villemure K, Farmer JP, Andermann F, Villemure JG. Posterior quadrantic epilepsy surgery: technical variants, surgical anatomy, and case series. Epilepsia l2007;48: 1429-37[Pubmed]
[15]]Daniel RT, Meagher-Villemure K, Roulet E, Villemure JG. Surgical treatment of temporoparietooccipital cortical dysplasia in infants: report of two cases. Epilepsia l2004;45: 872-6[Pubmed]
[16Delalande O, Pinard JM, Basdevant C, Gauthe M, Plouin P, Dulac O. Hemispherotomy. A new procedure for central disconnection. Epilepsia l1992;33 (suppl 3): 99–100. Abstract.
[17]Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia l2005;46: 561-7.[Pubmed]
[18]Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol l1998;44: 740-8[Pubmed]
[19]Czochanska J, Langner-Tyszka B, Losiowski Z, Schmidt-Sidor B. Children who develop epilepsy in the first year of life: a prospective study. Dev Med Child Neurol l1994;36: 345-50[Pubmed]
[20]]Huttenlocher PR, Hapke RJ. A follow-up study of intractable seizures in childhood. Ann Neurol l1990;28: 699-705.[Pubmed]
[21]Vasconcellos E, Wyllie E, Sullivan S, Stanford L, Bulacio J, Kotagal P, Bingaman W. Mental retardation in pediatric candidates for epilepsy surgery: the role of early seizure onset. Epilepsia l2001;42: 268-74.[Pubmed]
[22]Pulsifer MB, Brandt J, Salorio CF, Vining EP, Carson BS, Freeman JM. The cognitive outcome of hemispherectomy in 71 children. Epilepsia l2004;45: 243-54[Pubmed]
[23]Vining EP, Freeman JM, Pillas DJ, Uematsu S, Carson BS, Brandt J, Boatman D, Pulsifer MB, Zuckerberg A. Why would you remove half a brain? The outcome of 58 children after hemispherectomy-the Johns Hopkins experience: 1968 to 1996. Pediatrics l1997;100: 163-71.[Pubmed]
[24Asarnow RF, LoPresti C, Guthrie D, Elliott T, Cynn V, Shields WD, Shewmon DA, Sankar R, Peacock WJ. Developmental outcomes in children receiving resection surgery for medically intractable infantile spasms. Dev Med Child Neurol l1997;39: 430-40[Pubmed]
[25]Lettori D, Battaglia D, Sacco A, Veredice C, Chieffo D, Massimi L, Tartaglione T, Chiricozzi F, Staccioli S, Mittica A, Di Rocco C, Guzzetta F. Early hemispherectomy in catastrophic epilepsy: a neuro-cognitive and epileptic long-term follow-up. Seizure l2008;17: 49-63.[Pubmed]
[26]]Cross JH. Epilepsy surgery in childhood. Epilepsia l2002;43 Suppl 3: 65-70[Pubmed]
[27]Shields WD. Catastrophic epilepsy in childhood. Epilepsia l2000;41 Suppl 2: S2-6[Pubmed]
[28]Duchowny M, Jayakar P. Functional cortical mapping in children. Adv Neurol l1993;63: 149-54.[Pubmed]
[29]Gonzalez-Martinez JA, Gupta A, Kotagal P, Lachhwani D, Wyllie E, Luders HO, Bingaman WE. Hemispherectomy for catastrophic epilepsy in infants. Epilepsia l2005;46: 1518-25[Pubmed]
[30]Maehara T, Shimizu H, Shigetomo R, Tamagawa K. [Functional hemispherectomy for children aged 2 years or less for the treatment of intractable epilepsy caused by cortical dysgenesis]. No To Hattatsu l2000;32: 395-400[Pubmed]
[31].]Maehara T, Shimizu H, Kawai K, Shigetomo R, Tamagawa K, Yamada T, Inoue M. Postoperative development of children after hemispherotomy. Brain Dev l2002;24: 155-60.[Pubmed]
[32]Schropp C, Sorensen N, Krauss J. Early periinsular hemispherotomy in children with Sturge-Weber syndrome and intractable epilepsy--outcome in eight patients. Neuropediatrics l2006;37: 26-31[Pubmed]
[33]]Kubota M, Goishi K, Takemura S, Kawai K, Arai N. Early hemispherotomy in a patient with multilobar cortical dysplasia with intractable seizure: clinical-neurophysiological study. Eur J Paediatr Neurol l2008;12: 516-20.[Pubmed]
[34].Engel J, Jr. When is imaging enough? Epileptic Disord l1999;1: 249-53.[[Pubmed]
[35].] Freitag H, Tuxhorn I Cognitive functioning in preschool children after epilepsy surgery: rationale for early treatment. Epilepsia 2005;46 (4):561-567[Pubmed]

